Perspectives
Biosimilar strategy for infliximab by MedDrive™ includes the reference drug
This strategy strengthens infliximab’s position within the drug class
January 18, 2022TNF promotes inflammation, which plays an important role in the immune system. But when a person has one of these autoimmune diseases, their body makes too much TNF. Infliximab blocks the effect of the protein tumor necrosis factor-alpha.
Definition: White blood cells in the body produce tumor necrosis factor. It’s a protein that can trigger inflammation to help fight off infection.
Remicade’s (infliximab’s) FDA approvals
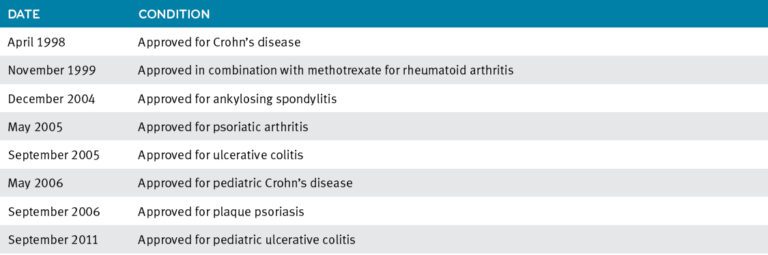
In a healthy person, the body knows how to block extra TNF. When someone has an autoimmune disease, the extra TNF creates problems. In RA, it causes joint swelling and redness. In psoriasis, high levels of TNF can lead to red, scaly patches on the skin.2
Infliximab and other TNF inhibitors improved the treatment of these diseases characterized by chronic inflammation. Since coming on the scene, TNF inhibitors have transformed treatment practices to help make remission become a realistic goal of therapy. For many patients with these conditions, that has meant that long-term disability from structural damage can be prevented.3
Other TNF Inhibitors

Remicade ranked as the 30th best-selling drug in 2020 with more than $3.7 trillion in 2020 U.S. sales.4 That leaves plenty of room for growth for infliximab biosimilars.
Infliximab and its biosimilars
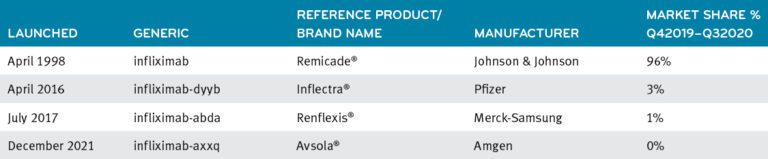 Use of infliximab has been on a modest decline of about 5.5% since 2018. Infliximab cost on a per member per month basis was $2.09 in 2020, primarily billed under the medical benefit.5
Use of infliximab has been on a modest decline of about 5.5% since 2018. Infliximab cost on a per member per month basis was $2.09 in 2020, primarily billed under the medical benefit.5
Every month, 6% of the patients using infiximab are new to therapy (called the “new start rate”). This comes from rises in utilization across many different conditions.
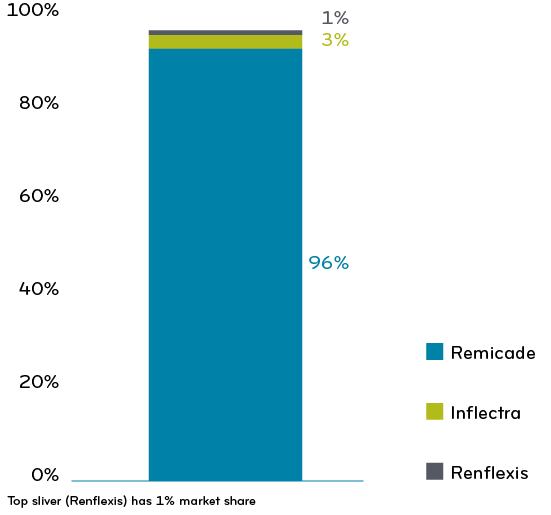
Infliximab market share 2019-2020
Infliximab is administered as an intraveneous (IV) infusion, primarily given in an outpatient hospital setting. Outpatient hospital settings have been shown to be more expensive than infusion centers or doctors’ offices. Moving to a less expensive site of care, like an ambulatory infusion center or doctor’s office, could save Prime’s Blue Plan clients well over $100 million.
Prime’s MedDrive recommendation: Include two biosimilars along with Janssen Biotech’s Remicade® (infliximab).
Most of Prime’s Blue Plan clients have benefit designs that prefer Remicade. We have recommended that they prefer two biosimilars and the reference product. These changes will be reflected in the online formularies.
MedDrive medical solutions is a complete toolkit
MedDrive leverages the collective strength of Prime’s client membership to help control medical costs. Client savings are obtained through:
- Improved contracts from manufacturers
- Lower net costs with shift of use from expensive medical drugs to lower cost alternatives, such as biosimilars
- Lower cost alternatives within the same therapeutic class
Prime’s Blue Plan clients are executing preferred biosimilar programs that will save them hundreds of millions of dollars. See Prime’s recommendations for bevacizumab, trastuzumab and rituximab. For more information, contact your local Prime representative.
References
- Remicade FDA Approval History. Drugs.com. Copyright © 2000-2021 Drugs.com. Accessed at: https://www.drugs.com/history/remicade.html
- “How does TNF cause inflammation?” By Hope Christol. August 25, 2020. WebMD. WebMD Medical Reference. © 2019 WebMD, LLC. All rights reserved. Accessed at: https://www.webmd.com/rheumatoid-arthritis/how-does-tnf-cause-inflammation
- Melsheimer, Richard & Geldhof, Anja & Apaolaza, Isabel & Schaible, Thomas. (2019). Remicade® (Infliximab): 20 years of contributions to science and medicine. Biologics: Targets and Therapy. Volume 13. 139-178. 10.2147/BTT.S207246. Accessed at. https://www.researchgate.net/publication/334768817_RemicadeR_Infliximab_20_years_of_contributions_to_science_and_medicine
- 50 of 2020’s best-selling pharmaceuticals, by Brian Buntz, May 14, 2021. Drug Discovery and Development © 2022 WTWH Media LLC. Accessed at: https://www.drugdiscoverytrends.com/50-of-2020s-best-selling-pharmaceuticals/
- Prime book of business data.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts