COST-EFFECTIVE SPECIALTY DRUG MANAGEMENT
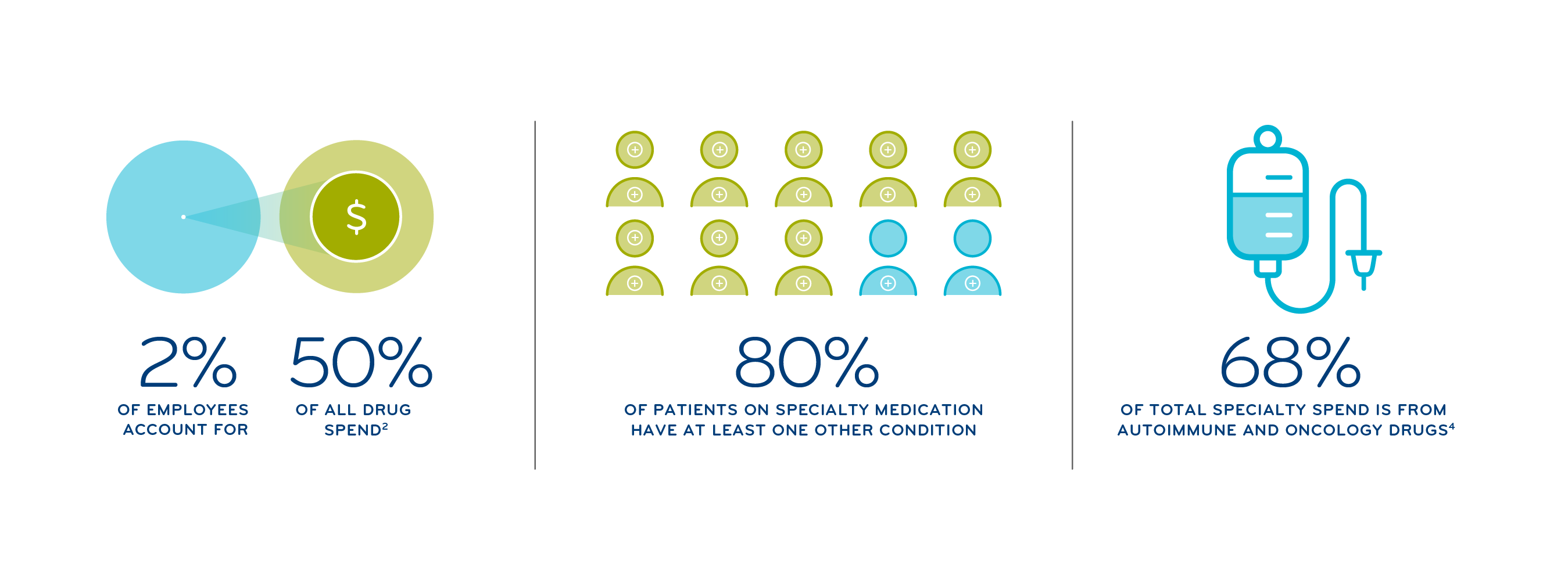
Today, specialty pharmacy accounts for more than a third of total health care costs, with specialty drugs making up 55% of drug spend — and a predicted 65% by 2025 due to a pipeline that’s two-thirds specialty drugs. 1,2
Watch VideoTHE PROBLEM
Specialty drug costs continue to rise for employers and employees
Specialty drugs are used to treat complex, chronic or rare medical conditions. They often have unique dosing, administration or monitoring requirements, and many specialty drugs are managed through the medical benefit, not pharmacy. What’s more, these high-cost drugs can run into the tens of thousands of dollars — and more — and a small number of employees can have a big impact on costs.
PROACTIVE APPROACH
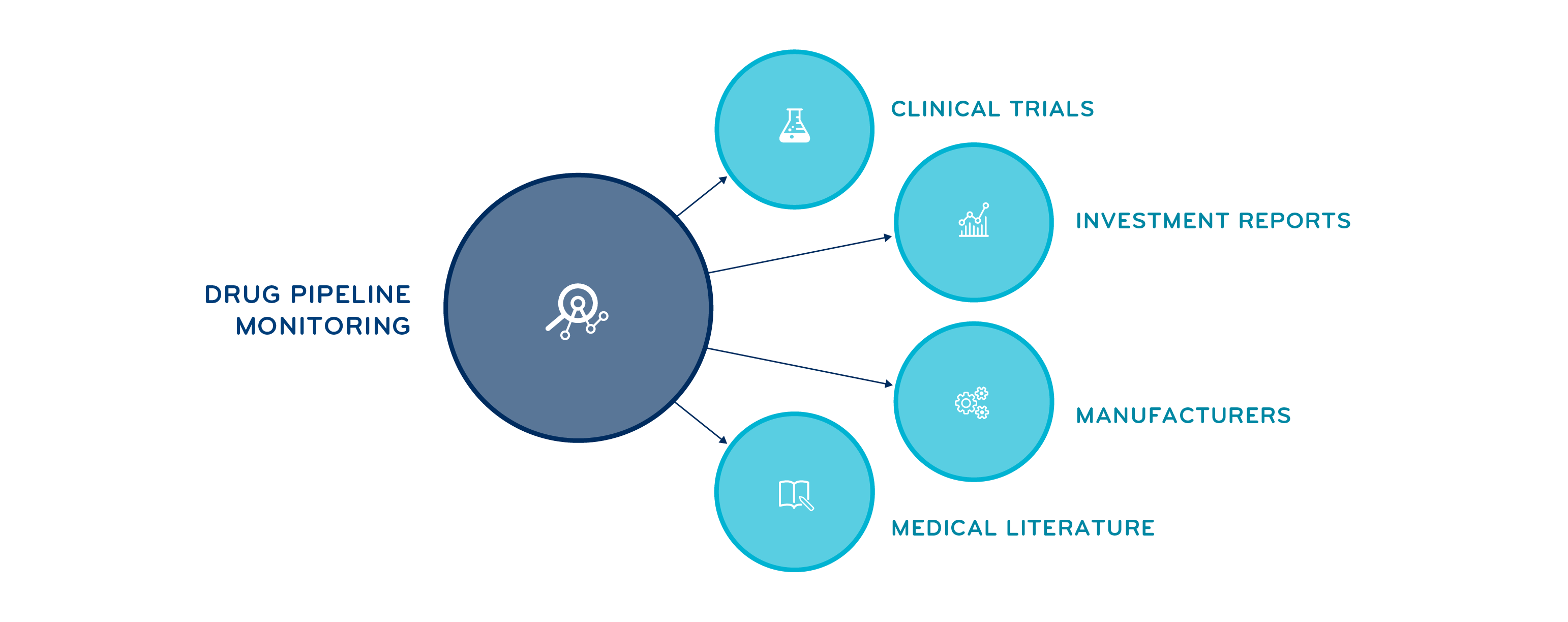
Planning through research and drug pipeline monitoring
Costly gene and cell therapies and biologic drugs used to treat complex conditions continue to dominate the drug pipeline. Prime and Magellan Rx uses specialized pharmacists and data scientists to research and monitor this pipeline, focusing on areas driving specialty trend, such as oncology and autoimmune.
CRUCIAL OPPORTUNITY
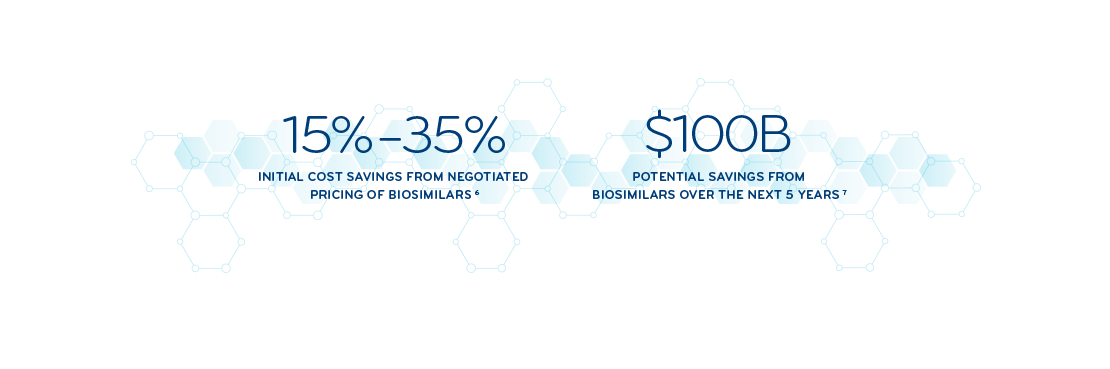
Embracing biosimilars to help lower drug costs
Biosimilars are highly similar to, and often less expensive than, their reference brand products, providing significant savings opportunities. Prime and Magellan Rx identifies biosimilars in the pipeline, allowing us to develop solutions that are ready from day one.
SPECIALTY SOLUTIONS
Navigating your options
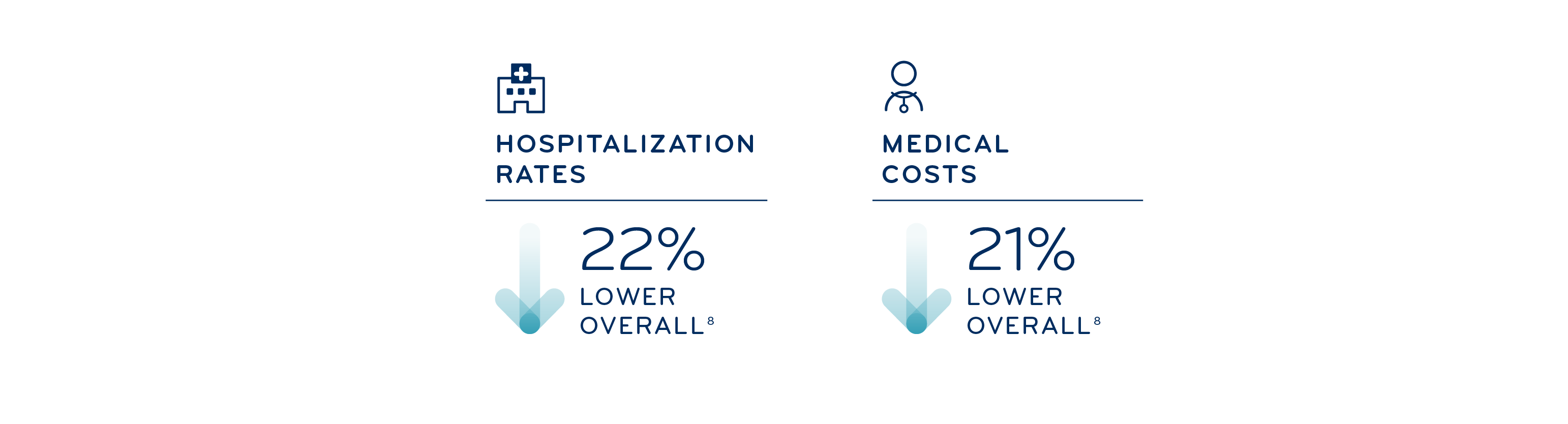
Now more than ever, employers need support in navigating the complicated and evolving specialty landscape. Research has shown that integrated solutions can lead to cost savings. That’s where Prime and Magellan Rx comes in, providing leading insights and a comprehensive suite of products to fit your needs, enhanced by our total drug management capabilities.
1 Pharmaceutical Strategies Group, LLC. (2022). 2022 Artemetrx state of specialty spend and trend report. https://www.psgconsults.com/specialtyreport2022
2 IQVIA Institute for Human Data Science. (2022, April). The use of medicines in the U.S. 2022. https://www.iqvia.com/insights/the-iqvia-institute/reports/the-use-of-medicines-in-the-us-2022
3 Jackson EA, et al. (2021). An analysis of member retention patterns for adult rare disease cohorts to support evaluating multiyear payment arrangements for novel therapies. JMCP 2021, 27(6), 753–759. https://www.jmcp.org/doi/pdf/10.18553/jmcp.2021.27.6.753
4 Fang, H., Frean, M., Sylwestrzak, G., & Ukert, B. (2022, February 1). Trends in disenrollment and reenrollment within US commercial health insurance plans, 2006-2018. JAMA Network Open, 2022, 5(2), e220320. doi:10.1001/jamanetworkopen.2022.0320
5 Prime book of business, 2023
6 U.S. Food and Drug Administration, and Federal Trade Commission. (2020, February 3). FDA and FTC Announce New Efforts to Further Deter Anti-Competitive Business Practices, Support Competitive Market for Biological Products to Help Americans [Press release]. https://www.fda.gov/news-events/press-announcements/fda-and-ftc-announce-new-efforts-further-deter-anti-competitive-business-practices-support
7 Cardinal Health. (2022, June 13). Humira® biosimilar landscape overview. https://www.cardinalhealth.com/en/product-solutions/pharmaceutical-products/biosimilars/humira-biosimilar-landscape-overview.html
8 Prime and BCBSMN internal analysis, 2022; compared to those not using specialty drugs and integrated benefits