Perspectives
Cell therapy growing quickly and serving rare conditions
FDA has approved a diverse group of cell and tissue therapies
May 9, 2023What is cell therapy?
Cell therapy involves the intravenous transfer of intact or biologically modified live cells or tissues to a patient. Cell therapy may prevent, treat, cure, diagnosis or mitigate rare cancers, diseases or injuries. It does so without altering the underlying genetic condition. The cells are cultivated or modified outside of the body before being injected into the patient. Cell therapy restores or alters certain sets of cells, or uses cells to carry a therapy throughout the body.1,2,3 Cells or tissues used in cell-based therapies may be obtained from the patient’s own cells or tissues (autologous) or from a donor (allogeneic).
CAR-T therapy, or chimeric antigen receptor T cell therapy, uses the technologies of both gene therapy and cell therapy. For Prime’s purposes, we define CAR-T as cell therapy, not gene therapy.
What is gene therapy? What’s the difference?
Gene therapy involves the transfer of genetic material, usually in a carrier or vector, and the uptake of the gene into the appropriate cells of the body. Cell therapy involves the transfer of cells with the relevant function into the patient. Some protocols use both gene therapy and cell therapy.
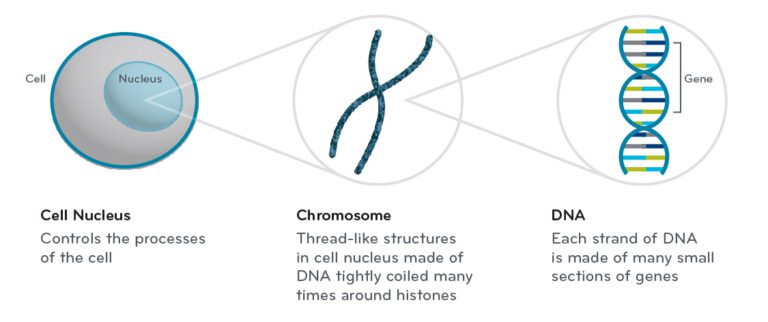
Cells are the basic building blocks of all living things; genes can be found deep within cells. Genes are small sections of DNA that carry genetic information and instructions for making proteins, which help build and maintain the body.1,2,3
Cell therapies on the market as of February 2023
There are seven cell therapy products FDA-approved to date. (See “What is gene therapy?” for a list of gene therapy products.)
An estimated five to 10 cell therapies have PDUFA dates in 2023.
FDA Approved CAR T-Cell Therapy One-Time Administration or One-Time Administration Tissue-Based Therapy with WAC of > $399,000
| Name | Manufacturer | Indication | WAC |
Approval Date* |
| Kymriah™ (tisagenlecleucel) | Novartis |
Pediatric ALL & LBCL & FL |
$508,250 $399,110 |
August 2017 |
|
Yescarta™ (axicabtagene ciloleucel) |
Kite | LBCL & FL | $399,000 | October 2017 |
|
Tecartus™ (brexucabtagene autoleucel) |
Kite | Mantle cell lymphoma | $399,000 | July 2020 |
|
Breyanzi™ (lisocabtagene maraleucel) |
BMS | LBCL | $410,300 | February 2021 |
|
Abecma™ (idecabtagene vicleucel) |
BMS and bluebird bio | Multiple myeloma | $419,500 | March 2021 |
| Rethymic™ (allogeneic processed thymus tissue agdc) | Enzyvant | Congenital athymia | ~$2.7 million | October 2021 |
|
Carvykti™ (ciltacabtagene autoleucel) |
Janssen | Relapsed and refractory multiple myeloma | $465,000 | February 2022 |
* Anticipated approval dates are predictions made by Prime Therapeutics, based on industry information.
References
- https://www.genemedi.net/i/ex-vivo-in-vivo-in-vitro
- https://asgct.org/education/more-resources/gene-and-cell-therapy-faqs#:~:text=Gene%20therapy%20involves%20the%20transfer,gene%20therapy%20and%20cell%20therapy.
- https://www.novartis.com/about/innovative-medicines/novartis-pharmaceuticals/novartis-gene-therapies/what-cell-and-gene-therapy#:~:text=Cells%20are%20the%20basic%20building,and%20maintain%20the%20body1.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts