Perspectives
Small in number, but large in impact, “drug super spenders” need super attention
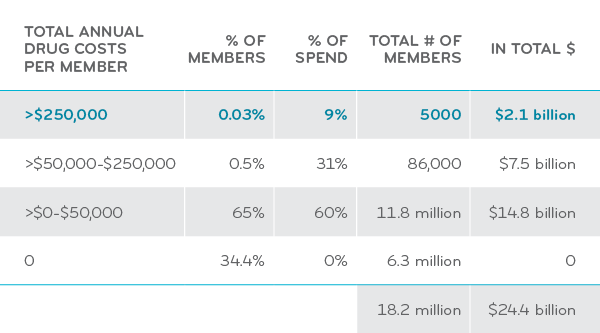
Just 0.03 percent of members have annual drug costs over $250,000. They account for 9 percent of total drug spend.
December 9, 2019Innovative gene-based therapies and ultra-expensive specialty drugs are bringing hope to people affected by rare diseases. At the same time, these drugs are often causing headaches and heartburn for families and employers struggling with health care bills exceeding hundreds of thousands, even millions of dollars, a year.
In 2018, members with over $250,000 a year in total drug costs accounted for 28 of every 100,000 commercially insured members. That’s just a tiny fraction – 0.03 percent – of Prime’s commercial members. We call them “drug super spenders” – and they accounted for nearly 9 percent of total drug spend across both medical and pharmacy benefits.
Finding these members requires deep knowledge and understanding of drug billing through both the medical and pharmacy benefits as well an integrated medical and pharmacy database; Prime has both.
As a group, drug super spenders are growing fast
The number of these drug super spenders with total annual drug costs over $250,000 has grown 63 percent over the last three years. And Prime is forecasting this growth rate will continue. In five years, we expect drug super spenders will account for more than 15 percent of total drug spend.
Number of members by drug cost range (2018 claims data)
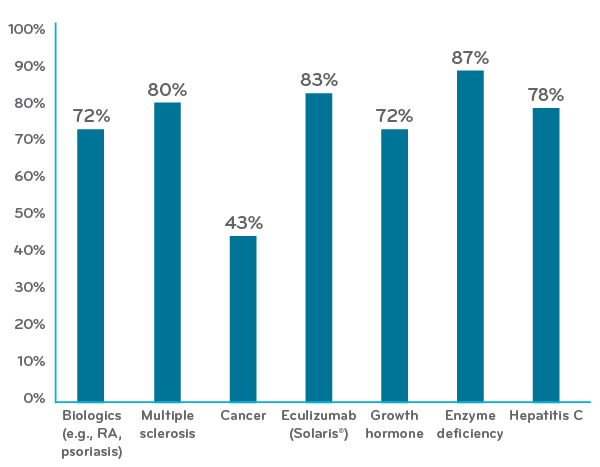
Specialty drugs for rare diseases cost hundreds of thousands of dollars per patient. While they aren’t as common as chronic conditions like diabetes or high cholesterol, all it takes is one employee with a specialty condition to significantly impact one employer’s total health care spend.
For many specialty conditions, nearly 90 percent of the total cost of care can come from drugs. That’s why Prime’s expertise at drug management is so important in controlling costs.
An interesting article in the December 2019 Specialty Pharmacy Continuum included some additional commentary about the challenges of managing the costs of these drug super spenders. The reporter quotes me and several other industry experts.1
Drug costs as a percentage of total care for some specialty conditions
The number of drugs serving drug super spenders is growing
The importance of high cost drugs is only going to grow. Of 59 new drugs approved in 2018, 34 were for rare diseases.2 The pipeline is full of drugs that treat ultra-rare diseases. Forecasts suggest that by 2025, we will start to see 10 to 20 new gene therapy drugs each year.3 Costs for these drugs is very high. Annual drug therapy cost for these ultra-rare drugs generally range from $250,000 to more than $1 million.
Prime’s approach to total drug cost management
Prime’s research on drug super spenders is a good example of how we put our total drug management strategy to work for the benefit of our clients and their members.
Our unique business model has allowed us to analyze integrated medical and pharmacy claims for 20 years. We understand how these benefits interact. We have a full view of how specialty drugs are being prescribed and used. We have successfully produced groundbreaking research on total cost of care by therapeutic condition. This has helped us develop an extensive toolkit of drug management strategies.
Integrated medical-pharmacy reporting: Prime has analytics that model potential client savings if various Prime products are implemented. The integrated modeling program uses plan-specific data to produce actionable insights.
- Cross benefit cost share: Because specialty drugs can be covered under either the medical benefit or the pharmacy benefit, Prime works with clients to structure a consistent drug cost share for members across benefits.
- Site of care: This program focuses on shifting infusion patients from high cost hospital outpatient facilities to lower cost sites such as the patient’s home, infusion center or doctor’s office. Optimizing the sites of care can generate an estimated savings of $1.41 PMPM.
- Reimbursement solutions: We review medical drug fee schedules to bring them in line with market competitive rates so that health plans are reimbursing for provider administered drugs in the most efficient and effective manner, and allowing for lower cost and/or preferred product options.
- Medical claim edits: We identify claims that are out of line with expected cost, quantity and clinical use for potential savings of $0.99 PMPM. One clinical product, GuidedHealth®, yielded a medical cost avoidance of nearly $500 million in 2018.
- Advanced FWA: Our integrated analytics use medical and pharmacy data to investigate fraud, waste and abuse (FWA) at the member, prescriber and pharmacy levels. Clinical products identify and eliminate FWA in all its forms. Then Prime’s special investigative unit (SIU) team audits and investigates any remaining suspicious claims. Prime saved clients $279 million through enhanced fraud, waste and abuse program last year.4
- Value-based contracting: Prime’s philosophy is to hold manufacturers accountable to the stated benefits of ultra-high-cost drugs. Prime currently has value-based contracts with manufacturers on several ultra-high cost therapies to manage orphan conditions, such as those seen in infants and young children.
Our cost management programs combine with therapeutic class/disease expertise. We use client-specific integrated medical and pharmacy claims analysis to confirm effectiveness of existing programs and to identify additional opportunities. Our collaborative approach with clients leads to market-specific solutions. Using the tools above, Prime presented $424 million in savings opportunities for clients in 2018.5 We need to do more. We have to do more.
References
- “‘Super Spenders’ Skyrocketing,” by Alison McCook. Specialty Pharmacy Continuum. December 9, 2019. Copyright © 2004-2019 McMahon Publishing. Specialty Pharmacy Continuum and SpecialtyPharmacyContinuum.com are part of McMahon Publishing. Accessed at: https://www.specialtypharmacycontinuum.com/Policy/Article/12-19/-Super-Spenders-Skyrocketing/56547
- “The $6 Million Drug Claim,” By Katie Thomas and Reed Abelson. Aug. 25, 2019. New York Times. All rights reserved. Accessed at: https://www.nytimes.com/2019/08/25/health/drug-prices-rare-diseases.html2.
- FDA Statement. Food and Drug Administration, FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D. Jan. 15. 2019 Accessed at: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics
- Page 4. Prime’s Focus on Trend Spring 2019 Trend Report-Commercial. Published Spring 2019 on 2018 data. Accessed at: https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2019/document-commercial-trend-spring-2019.pdf
- https://www.primetherapeutics.com/en/news/pressreleases/2019/release-infographic-fwa.html
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts