Perspectives
Quarterly Drug Pipeline
April 30, 2024Clinical insights and competitive intelligence on anticipated drugs in development
Drug pipeline
Clinical insights and competitive intelligence on anticipated drugs in development.
Table of contents
Editor-in-chief’s message
Welcome to the Prime Therapeutics + Magellan Rx Quarterly Pipeline! Dive into clinical insights and competitive intelligence on anticipated drugs in development, so you are well-sourced on the drug pipeline.
METHODOLOGY
The drug pipeline is complex and fluid. Our talented and committed team of clinical and analytics experts are excited to bring you this robust publication after thoughtful research. Specialty and traditional drugs that are covered under the pharmacy and medical benefits are featured. New molecular entities, pertinent new and expanded indications for existing medications, biosimilars and regenerative medicines, such as gene and cellular therapies, are also profiled.
Quarterly Pipeline details both agents submitted for United States (U.S) Food and Drug Administration (FDA) review and those in phase 3 study with a likelihood to apply to the FDA. Our Deep Dives consider the evidence, the products’ potential to fill an unmet need or become the new standard of care, and the ability to replace existing therapies.
A market agnostic financial forecast primarily from Evaluate™ is included for select agents to assist payers with assessing the potential budgetary impact of the pipeline. Five-year projected annual U.S. sales are forecasted.
REFLECTION
Thus far in 2024, the agency has approved 11 novel drugs which is on par with the number of approvals about the same time last year. Of note, most of the approvals so far in 2024 use at least one of the FDA’s expedited approval programs. Some of the noteworthy approvals include a new agent for NASH, a new indication for Wegovy® for secondary CVD prevention in obesity/overweight, a new option for Duchene muscular dystrophy and a new medication for pulmonary arterial hypertension. While numbers do not tell the entire story, they do represent significant innovation in patient care and advance public health for the American public.
ON THE HORIZON
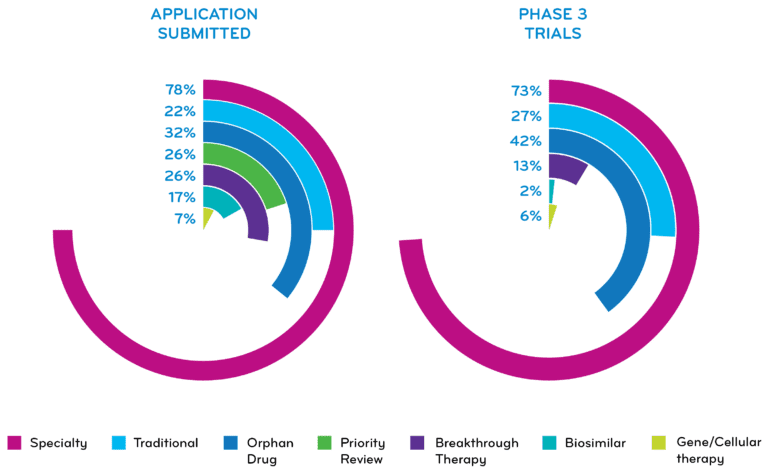
The FDA decisions for specialty medications (78%) and for orphan drugs (32%) continue to grow for agents with applications submitted to the FDA. Two agents are seeking FDA’s Accelerated Approval.
Notable anticipated approvals for second quarter 2024 include:
- A gene therapy for a rare disease
- First biologic for COPD
- First biosimilar to intravitreal Eylea®
- First-in-class oncology agent for SCLC
- First RSV vaccine using the mRNA platform
We hope you enjoy the report!
Maryam Tabatabai
Vice President, Clinical Information
Editorial team
Maryam Tabatabai, PharmD
Editor-In-Chief
Vice President, Clinical Information
Carole Kerzic, RPh
Executive Editor
Clinical Pharmacist, Drug Information
Nicole Kjesbo, PharmD, BCPS
Executive Editor
Clinical Program Director, Pipeline
Consultant Panel
Robert Greer, RPh, BCOP
Vice President, Clinical Strategy and Programs
Andrea Henry, PharmD, MBA, BCPS
Specialty Drug Information Pharmacist
Katie Lockhart
Senior Manager, Analytics
Olivia Pane, PharmD, CDCES
Clinical Pharmacist, Drug Information
The drug pipeline is fluid; the dates and information within this publication are subject to change. Nothing herein is or shall be construed as a promise or representation regarding past or future events and Prime Therapeutics/Magellan Rx Management expressly disclaims any and all liability relating to the use of or reliance on the information contained in this presentation. The information contained in this publication is intended for educational purposes only and should not be considered clinical, financial, or legal advice. By receipt of this publication, each recipient agrees that the information contained herein will be kept confidential and that the information will not be photocopied, reproduced, distributed to, or disclosed to others at any time without the prior written consent of Prime Therapeutics/Magellan Rx Management.
Pipeline
Deep dive
The Deep Dive section features an analysis for select pipeline drugs that are expected to be FDA-approved in the upcoming quarters. Selected agents are expected to have a high clinical and/or financial impact on healthcare. Agent selection considers the evidence — the products’ potential to fill an unmet need, become the new standard of care, or the ability to replace existing therapies. Typically, Deep Dives focus on new moieties, however, existing drugs that may offer a novel mechanism for existing conditions may be featured. Agents granted designations from the FDA to expedite review and/or support development and evaluation of pipeline drugs are also considered.
Specialty drug names appear in magenta throughout the publication.
Oncology/Cellular — afamitresgene autoleucel
afamitresgene autoleucel IV
Manufacturer: Adaptimmune
PROPOSED INDICATIONS
Advanced synovial sarcoma
CLINICAL OVERVIEW
Mechanism of action
Afamitresgene autoleucel (afami-cel) is an autologous T-cell therapy transduced via a lentiviral vector to express a high-affinity and specific T-cell receptor that targets melanoma-associated antigen A4 (MAGE-A4) peptides expressed along with human leukocyte antigen-A2 (HLA-A2) on the tumor cell surface.
Clinical trials
Afami-cel is being evaluated in the ongoing, open-label, single-arm, phase 2 SPEARHEAD-1 trial (NCT04044768) in patients with metastatic or inoperable advanced synovial sarcoma. Enrolled patients are 16 to 75 years of age (10 years of age at select sites) and have tumors that are HLA-A*02 and MAGE-A4 positive. Patients received previous treatment with an anthracycline agent or ifosfamide, with a median of three prior lines of systemic therapy (range, 1 to 12). An interim analysis reported an overall response rate (ORR) of approximately 39% (primary endpoint) and a median duration of response (DOR) of about 12 months. The median overall survival (OS) with afami-cel was higher compared to the historical OS in patients who received ≥ two prior lines of therapy (approximately 17 months versus < 12 months, respectively), and 70% of patients were alive two years after receiving afami-cel. Treatment-emergent adverse events (TEAEs) with afami-cel included cytokine release syndrome (CRS) and hematologic toxicities.
Dosage and administration
Afami-cel is administered via IV infusion as a single dose of 1 x 109 to 10 x 109 transduced T cells after receiving lymphodepleting chemotherapy (cyclophosphamide and fludarabine).
PLACE IN THERAPY
Synovial sarcoma is a rare soft tissue sarcoma (STS), with about 800 to 1,000 new cases occurring each year in the U.S. It is a mesenchymal tumor caused by translocation between chromosomes X and 18 that results in the expression of several SS18-SSX fusion proteins. Synovial sarcoma is primarily diagnosed in adolescents and adults < 30 years of age. It can occur anywhere in the body but usually develops near joints in the extremities (hip, knee, ankle and shoulder). Synovial sarcoma is an aggressive tumor with a high likelihood for metastasis. The overall five-year survival rate is estimated between 36% to 76%, and depends on tumor size, location and tumor spread.
Surgical resection with radiation therapy is the standard of care (SOC) for localized synovial sarcoma. Neoadjuvant or adjuvant chemotherapy (anthracycline-based regimen ± ifosfamide) is also used and is associated with an estimated response rate of 25% to 60%. Certain tyrosine kinase inhibitors are recommended in the presence of NTRK gene mutation (larotrectinib [Vitrakvi®], entrectinib [Rozelytrek®]) or in the advanced or metastatic setting (pazopanib [Votrient®]).
MAGE-A4 is present in up to 82% of synovial sarcoma tumors and is not expressed in normal tissue, making it a potential target for treatment. Afami-cel is a first-in-class T-cell receptor T-cell therapy directed at MAGE-A4 and has demonstrated benefit in treating advanced cases. If approved, it may prove to be an important treatment for patients with advanced synovial sarcoma, a population with poor prognosis and limited options.
FDA APPROVAL TIMELINE
August 4, 2024
FDA designations: Orphan Drug, Priority Review, RMAT
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected annual U.S. sales | $3 | $9 | $15 | $20 | $26 |
Respiratory – Dupixent®
Dupixent® (dupilumab) SC
Manufacturer: Sanofi/Regeneron
PROPOSED INDICATIONS
Uncontrolled chronic obstructive pulmonary disease (COPD) as add-on maintenance therapy.
CLINICAL OVERVIEW
Mechanism of action
Dupixent® is a human monoclonal antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling.
Clinical trials
The randomized, double-blind, 52-week, phase 3 BOREAS trial (NCT03930732; n=939) evaluated the safety and efficacy of Dupixent in adults with COPD and evidence of type 2 inflammation (eosinophil count ≥ 300 cells/µL). COPD was uncontrolled despite triple therapy (ICS + LABA + LAMA). Eligible patients were 40 to 80 years of age, current or former tobacco smokers with a smoking history of ≥ 10 pack-years and did not have a current or past diagnosis of asthma. In the trial, Dupixent led to a significant 30% reduction in the primary endpoint of annualized rate of moderate or severe COPD exacerbations compared to placebo (0.78 versus 1.1, respectively; rate ratio, 0.7; p<0.001). Per the study protocol, moderate exacerbations required treatment with a systemic glucocorticoid and/or an antibiotic agent, and severe exacerbations led to hospitalization, emergency medical care or death. Topline data from the replicate, phase 3 NOTUS study (NCT04456673, n=935; 40 to 85 years of age) confirmed the results of the BOREAS trial and reported a 34% reduction in annualized rate of moderate or severe COPD exacerbations at 52 weeks (p=0.0002) with Dupixent compared to placebo. In addition, in both trials, secondary endpoints showed significant improvements in lung function (FEV1) by week 12 that were sustained through week 52. Both trials reported a safety profile with Dupixent similar to its other approved indications. Common TEAEs reported were back pain, COVID-19, diarrhea, headache and nasopharyngitis.
Dosage and administration
In both trials, Dupixent was administered subcutaneously (SC) at a dose of 300 mg every two weeks.
PLACE IN THERAPY
An estimated 14.2 million (6.5%) people are diagnosed with COPD in the U.S., where it is a leading cause of death. COPD is a complex, chronic inflammatory airway disease characterized by respiratory symptoms (e.g., dyspnea, cough, sputum production and/or exacerbations) due to airway (bronchitis/bronchiolitis) and/or alveoli (emphysema) abnormalities that cause persistent, often progressive, airflow obstruction. A key risk factor for developing COPD is long-term exposure to toxic substances, such as tobacco smoke.
Type 2 inflammation is found in patients with asthma, and there is growing evidence that it is present in 20% to 40% of patients with COPD. The cytokines IL-4, IL-5 and IL-13 play a key role in type 2 inflammation by promoting the activation and trafficking of type 2 inflammatory cells, including eosinophils, in the lungs. Type 2 inflammation may increase the risk of COPD exacerbation, although evidence is mixed. Moreover, tobacco smoking has been shown to increase the production of pro-inflammatory cytokines (e.g., IL-4, IL-8, TNF-α) and may lead to a decreased response to inhaled corticosteroids, which is recommended as an add-on therapy in patients with severe COPD with a history of exacerbations.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends triple therapy with a LABA + LAMA + ICS for patients who have experienced ≥ 2 moderate exacerbations or ≥ 1 exacerbation leading to hospitalization if the blood eosinophil count is ≥ 300 cells/µL. For patients who experience a COPD exacerbation while on triple therapy, the addition of the oral PDE4 inhibitor Daliresp (roflumilast) (in patients with FEV1 < 50% predicted and chronic bronchitis) or the macrolide antibiotic azithromycin (for current non-smokers) may be considered.
Dupixent inhibits IL-4 and IL-13 signaling and thereby decreases type 2 inflammation. In phase 3 trials, when added to background triple therapy, Dupixent led to a significant reduction in moderate to severe COPD exacerbations in patients who were current or past smokers and had an elevated blood eosinophil level (≥ 300 cells/µL). If approved, this SC injectable agent will be the first biologic therapy indicated for COPD and could compete with Daliresp® as an add-on to triple therapy. Ensifentrine, an inhaled dual PDE3 and PDE4 inhibitor by Verona Pharma, has also been submitted for maintenance therapy in patients with moderate to severe COPD; an FDA decision is expected in late June 2024.
Dupixent is also approved for several other indications including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis (EoE) and prurigo nodularis (PN).
FDA APPROVAL TIMELINE
June 27, 2024
FDA designations: Breakthrough Therapy, Priority Review
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected Yearly U.S. Sales reported for COPD only |
$0 | $49 | $100 | $152 | $203 |
Immunology/Primary biliary cholangitis (PBC) agents – elafibranor + seladelpar
elafibranor oral
Manufacturer: Genfit / Ipsen
PROPOSED INDICATIONS
Primary biliary cholangitis (PBC)
CLINICAL OVERVIEW
Mechanism of action
Elafibranor is a dual peroxisome proliferator-activated receptor alpha and delta (PPAR-α/δ) agonist
Clinical trials
The randomized (2:1), double-blind, placebo-controlled phase 3 ELATIVE trial (NCT04526665) evaluated the efficacy and safety of elafibranor in 161 patients with PBC who had an inadequate response to ursodeoxycholic acid (UDCA). The primary endpoint was a biochemical response (defined as an alkaline phosphatase [ALP] level of < 1.67 × ULN, with a reduction of ≥ 15% from baseline, and normal total bilirubin levels) at week 52. This was achieved in 51% of patients who received elafibranor compared to 4% who received placebo (difference, 47%; p<0.001). There was no significant difference in resolution of moderate to severe pruritus between the groups. The most common TEAEs (≥ 10%) were abdominal pain, diarrhea, nausea and vomiting.
Dosage and administration
In the clinical trial, elafibranor 80 mg was administered orally once daily.
FDA APPROVAL TIMELINE
June 10, 2024
FDA designations: Breakthrough Therapy, Orphan Drug, Priority Review
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected Yearly U.S. Sales | $8 | $30 | $57 | $77 | $99 |
seladelpar oral
Manufacturer: Cymabay
PROPOSED INDICATIONS
Primary biliary cholangitis (PBC), including pruritus, in adults without cirrhosis or with compensated cirrhosis (Child Pugh A) who are inadequate responders or intolerant to UDCA
CLINICAL OVERVIEW
Mechanism of action
Seladelpar is a selective peroxisome proliferator-activated receptor-delta (PPAR-δ) agonist
Clinical trials
The randomized, double-blind, placebo-controlled phase 3 ENHANCE trial (NCT03602560) evaluated the efficacy and safety of seladelpar in 265 patients with PBC who had an inadequate response or intolerance to UDCA. Patients were randomized 1:1:1 to seladelpar 5 mg, seladelpar 10 mg, or placebo. The original primary endpoint was a composite biochemical response (defined as ALP < 1.67 × ULN, ≥ 15% ALP decrease from baseline, and total bilirubin ≤ ULN) at month 12. However, due to an erroneous safety signal (fully reversible and asymptomatic grade 3 aminotransferase increase) in a concurrent NASH trial, the ENHANCE trial was stopped early. At month 3, the mean duration of exposure was 17.7 weeks. At this timepoint a biochemical response was achieved in 57.1% and 78.2% of patients who received seladelpar 5 mg and 10 mg, respectively, compared to 12.5% who were given placebo (p<0.0001 for both doses). In addition, significantly more patients treated with seladelpar 10 mg had a significant reduction in pruritus compared to the placebo group (p=0.02). The most common TEAEs (≥ 5%), reported more often with seladelpar than placebo were upper abdominal pain and nausea.
Interim data from the similarly designed phase 3 RESPONSE trial (NCT04620733; n=193) reported at month 12, 61.7% of patients treated with seladelpar 10 mg achieved the primary composite response endpoint compared to 20% who received placebo (p<0.0001). In addition, a significant improvement in pruritus with seladelpar 10 mg compared to placebo was reported at month 6 and maintained through month 12.
The open-label ASSURE trial (NCT03301506) reported long-term safety and efficacy of seladelpar 5 mg and 10 mg for up to 2 years; the study was terminated early due to erroneous safety signals in a concurrent NASH trial. No serious TEAEs were reported among the 106 patients treated with seladelpar for 2 years. Response rates increased from years 1 to 2 for the composite endpoint (ALP <1.67 × ULN, ≥ 15% decrease in ALP, and total bilirubin ≤ ULN) from 66% to 79%.
Dosage and administration
In the clinical trials, seladelpar 5 mg and/or 10 mg were administered orally once daily.
PLACE IN THERAPY
PBC is a rare, progressive, autoimmune liver disease in which bile ducts are gradually destroyed. In PBC, bile acids accumulate causing damage to hepatocytes, and if left untreated, can lead to liver failure and death. Common symptoms of PBC include fatigue and pruritus, which can be debilitating. The majority of PBC cases occur in women 30 to 60 years of age. In the U.S., the prevalence is 654 per 1 million persons for women and 121 per 1 million persons for men. Genetic predisposition and environmental triggers have been associated with PBC and highly specific autoantibodies (antimitochondrial antibodies) are detected in most cases. Notably, elevated serum ALP and total bilirubin levels are biomarkers associated with poor clinical outcomes in PBC.
UDCA (Urso Forte), a bile salt, is approved for the treatment of PBC. It protects the liver against toxic bile salts and inhibits apoptosis and fibrosis. If started early in the disease process, UDCA may delay disease progression. The oral farnesoid X receptor agonist, obeticholic acid (Ocaliva®), is approved as add-on therapy for patients with an inadequate response to UDCA, which accounts for up to 40% of patients treated with UDCA. While obeticholic acid may improve laboratory measures (ALP, GGT, transaminase levels), it does not improve survival or PBC symptoms. Off-label use of oral fibrates can also be considered as an alternative for patients without decompensated liver disease who do not adequately respond to UDCA. Methotrexate, cyclosporine, colchicine and corticosteroids have also been used.
The peroxisome proliferator activator receptor (PPAR) is a nuclear receptor involved in a variety of metabolic processes, including bile acid homeostasis. It has three isoforms: α, δ and γ. PPAR-α is primarily expressed in the liver and regulates bile acid synthesis and detoxification, phospholipid secretion, and inflammatory pathways. PPAR-δ and PPAR -γ are more universally found in metabolic active tissues and when activated, they affect lipid and glucose metabolism, and exhibit anti-inflammatory and anti-fibrotic properties. While seladelpar stimulates PPAR- δ, elafibranor acts on both PPAR- α and PPAR-δ. In clinical trials, elafibranor and seladelpar were well tolerated and demonstrated a significant biochemical response. However, while seladelpar led to a marked improvement in pruritus, the characteristic symptom of PBC, the same was not true for elafibranor. If approved, these agents will be the first PPAR agonists available to treat PBC in patients with an inadequate response to UDCA and will be additional therapeutic options to Ocaliva.
FDA APPROVAL TIMELINE
August 14, 2024
FDA designations: Breakthrough Therapy, Orphan Drug, Priority Review
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected Yearly U.S. Sales | $12 | $114 | $220 | $364 | $533 |
Oncology – imetelstat
imetelstat IV
Manufacturer: Geron
PROPOSED INDICATIONS
Transfusion-dependent anemia in patients with lower-risk myelodysplastic syndromes (MDS) who have failed to respond to, have lost response to, or are ineligible for erythropoiesis-stimulating agents (ESAs)
CLINICAL OVERVIEW
Mechanism of action
Imetelstat is a telomerase inhibitor. It selectively targets malignant hematopoietic stem and progenitor cells with high telomerase activity by direct binding to the RNA template, resulting in malignant cell apoptosis and potential disease-modifying activity.
Clinical trials
The randomized, double-blind, placebo-controlled, phase 3 IMerge trial (NCT02598661) evaluated imetelstat in 178 adults with RBC transfusion-dependent, low- or intermediate-risk MDS that was relapsed, refractory to, or ineligible for ESA therapy. Patients had not received prior treatment with azacitidine, decitabine, or lenalidomide. The median follow-up was 19.5 months in the imetelstat group and 17.5 months in the placebo group. The study demonstrated that significantly more patients treated with imetelstat achieved the primary endpoint of RBC transfusion independence in ≥ 8 weeks compared to those who were given placebo (40% versus 15%, respectively; p=0.0008). In addition, 28% and 13.6% of patients treated with imetelstat remained RBC transfusion-independent at ≥ 24 weeks and ≥ 1 year, respectively, compared to 3.3% and 1.7%, respectively, in those who received placebo. Grade 3 to 4 TEAEs occurred in 91% of patients in the imetelstat group compared to 47% in the placebo group. Of these, neutropenia and thrombocytopenia were the most common, and occurred most often during the first three treatment cycles. Cytopenia was managed with treatment delays and dose adjustments in accordance with the study protocol.
Dosage and administration
In the clinical trial, imetelstat 7.5 mg/kg was administered IV over 2 hours every four weeks until disease progression or unacceptable toxicity occurred.
PLACE IN THERAPY
In MDS, the bone marrow fails to produce healthy blood cells, leading to cytopenia. The incidence rate of MDS is approximately 4.5 per 100,000 people per year in the general population; however, individuals over 60 years of age are most commonly affected. About 90% of cases are primary or idiopathic in nature, resulting from age-related injury to stem cells. The remaining cases are the result of cytotoxic chemotherapy or radiation therapy. The majority of patients (85%) with MDS experience fatigue or shortness of breath due to underlying anemia. Neutropenia and thrombocytopenia usually occur during advanced stages of the disease. Once termed preleukemia, MDS evolves into acute myeloid leukemia (AML) in about 30% of patients.
HSCT is the only available curative treatment for MDS. Allogeneic HSCT is considered in transplant candidates with intermediate or greater risk disease. When HSCT is not appropriate, an alternative therapy is RBC transfusions. Pharmacologic agents include ESAs (e.g., epoetin alfa, darbepoetin) and the erythroid maturation agent luspatercept-aamt (Reblozyl®; for sideroblastic MDS). Agents used to slow progression of the disease include hypomethylating agents (e.g., azacytidine, decitabine) and immune therapy (e.g., lenalidomide). In addition, use of investigational drugs in clinical trials remains an important aspect of MDS management.
Imetelstat is a first-in-class telomerase inhibitor. If approved, imetelstat would provide a new mechanism of action leading to durable transfusion independence when treating MDS-related anemia after treatment with ESAs has failed or in patients who are ESA ineligible. This agent could compete with Reblozyl in patients with ring sideroblastic MDS. On March 14, 2024, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor (12:2) of imetelstat for the treatment of anemia for low-risk MDS patients who are refractory or intolerant to ESAs.
Imetelstat is also in phase 3 trials for myelofibrosis and phase 2 trials for AML.
FDA APPROVAL TIMELINE
June 14, 2024
FDA designations: Fast Track, Orphan Drug
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected annual U.S. sales | $54 | $213 | $350 | $467 | $566 |
Behavioral health – midomafetamine
midomafetamine oral
Manufacturer: Lykos
PROPOSED INDICATIONS
Post-traumatic stress disorder (PTSD)
CLINICAL OVERVIEW
Mechanism of action
Midomafetamine (MDMA) is a psychoactive entactogen. It promotes the release of monoamines and hormones in the brain, including serotonin, norepinephrine, dopamine, oxytocin and cortisol that modulate emotional memory circuitry.
Clinical trials
Two multi-site, double-blind, phase 3 trials, MAPP1 (NCT03537014; n=90) and MAPP2 (NCT04077437; n=104), evaluated MDMA in patients with severe or moderate to severe PTSD, respectively. Patients were randomized 1:1 to MDMA or placebo, each used in combination with psychotherapy. The primary endpoint in both trials was change from baseline in the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) total severity score at week 18; a ≥ 10-point reduction in CAPS-5 total severity score was considered clinically meaningful. The mean baseline CAPS-5 total severity score was 39 points in MAPP1 and 44.1 points in MAPP2. Both trials met the primary endpoint, demonstrating a significant improvement in the CAPS-5 total severity score with MDMA compared to placebo (MAPP1: LS mean change, -4.4 versus -13.9, respectively [p<0.001]; MAPP2: LS mean change, -23.7 versus -14.8, respectively; [p<0.001]). In MAPP1, 67% of patients treated with MDMA no longer met the diagnostic criteria for PTSD and 33% achieved PTSD remission, compared to 32% and 5% of patients, respectively, who were given placebo. A similar trend was reported based on exploratory data in MAPP2. In addition, there was a significant improvement in the key secondary endpoint of Sheehan Disability Scale (SDS) functional impairment score from baseline to week 18 with MDMA compared to placebo (MAPP-1: LS mean change, -3.1 versus -2; respectively [p=0.0116]; MAPP-2: LS mean change, -3.3 versus -2.1; respectively [p=0.03]). In MAPP1, one and three patients in the MDMA and placebo groups, respectively, did not complete therapy due to adverse effects or treatment distress. In MAPP2, the dropout rate was 1.9% in the MDMA group and 15.7% in the placebo group. Suicidal ideation was not increased or intensified by MDMA. No cardiac events indicating QT prolongation occurred with MDMA. In addition, no potential for abuse of MDMA was reported.
Patients in the phase 3 trials were allowed to participate in a long-term follow-up study and were assessed ≥ 6 months after their last therapy session with MDMA or placebo. Interim data revealed durable improvements in CAPS-5 total severity score ≥ 6 months after the last dosing session, and for over a year among those who were followed for more than a year.
Dosage and administration
The treatment period in each trial consisted of three 8-hour experimental sessions during which patients received psychotherapy in addition to oral MDMA or placebo. The sessions were conducted approximately four weeks apart. At session 1, MDMA was given as 80 mg, followed by a supplemental 40 mg dose 1.5 to 2 hours later. In sessions 2 and 3, MDMA was given as 120 mg, followed by a supplemental 60 mg dose 1.5 to 2 hours later.
PLACE IN THERAPY
PTSD is a psychiatric condition that can occur in a person of any age who experiences or witnesses a traumatic event or series of events with a threat of injury or actual injury to themselves or others. It is estimated that PTSD affects about 8 to 12 million people in the U.S. in a given year, with a higher likelihood in women and minority groups. Symptoms of PTSD are severe enough to interfere in daily life. To meet the DSM-5 diagnosis for PTSD, all of the following symptoms must be present for at least one month: re-experiencing the event (e.g., flashback, nightmares), avoidance of trauma-related stimuli, arousal and reactivity symptoms (e.g., irritability, insomnia, easily startled, engaging in risky behavior), and cognition and mood symptoms (e.g., negative thoughts and emotions, feeling isolated). PTSD symptoms typically begin within 3 months of the traumatic event, but can present at any time, even decades after the event. PTSD is also associated with comorbidities, such as depression, anxiety, substance use and abuse, dissociative disorders, and harm toward self and others.
Trauma-focused psychotherapy aids in processing and moderating memories associated with trauma using in vivo and/or imaginal exposure. Trauma-focused psychotherapy is the first-line treatment for PTSD and includes techniques such as cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), cognitive therapy (CT), prolonged exposure therapy (PE), brief eclectic psychotherapy (BEP), eye movement desensitization and reprocessing (EMDR) and narrative exposure therapy (NET). While trauma-focused psychotherapy has proven to be effective, up to 50% of patients treated have lasting symptoms and nearly 25% do not complete therapy. Medication therapy (e.g., SSRIs) is an alternative first-line treatment in patients with comorbidities (e.g., depression, anxiety) or who are unable to participate in psychotherapy. Paroxetine (Paxil®) and sertraline (Zoloft®) are FDA-approved for PTSD; efficacy rates of ≤ 60% and remission rates of < 30% have been reported for these agents. Fluoxetine (Prozac®) and venlafaxine (Effexor® XR) are also recommended off-label. Evidence for combined psychotherapy and pharmacotherapy compared with psychotherapy alone is mixed.
MDMA promotes the release of monoamines and hormones in the brain that modulate emotional memory circuitry. Neuroimaging findings suggest that MDMA acts in key neural pathways responsible for memory and emotional processing (e.g., amygdala, hippocampus, prefrontal cortex). MDMA appears to facilitate reprocessing of traumatic memories with clearer recall and empathy for oneself, and without anxiety, emotional numbing or dissociation. In clinical trials, MDMA + trauma-focused psychotherapy led to a significant and durable improvement in PTSD symptoms compared to placebo, when administered in a setting designed to enhance and support the therapeutic effects of the agent. MDMA was well-tolerated, without increase in risk in suicidality, CV effects or abuse potential of the agent.
If approved, MDMA would be the first FDA-approved psychedelic agent and will be used in combination with psychotherapy for PTSD. Also known as ecstasy, MDMA is currently a Schedule I Controlled Substance in the U.S. and will require rescheduling by the DEA to allow for prescription use. Notably, states could have unique regulations to legalize and/or govern use. Another psychedelic agent, psilocybin, is also in clinical trials for PTSD (phase 2), as well as MDD (phase 3) and eating disorders (phase 2).
FDA APPROVAL TIMELINE
August 11, 2024
FDA designations: Breakthrough Therapy, Priority Review
FINANCIAL FORECAST (reported in millions)
The projected total U.S. sales for MDMA are not available.
Immunology – nemolizumab
nemolizumab SC
Manufacturer: Galderma
PROPOSED INDICATIONS
- Prurigo nodularis
- Moderate to severe atopic dermatitis
CLINICAL OVERVIEW
Mechanism of action
Nemolizumab is a monoclonal antibody targeting interleukin-31 receptor alpha (IL-31α), which blocks signaling from IL-31.
Clinical trials
The randomized, double-blind, phase 3 OLYMPIA 2 trial (NCT04501679) evaluated nemolizumab in 274 adults with moderate to severe prurigo nodularis. The primary endpoints were itch response, defined as a reduction of ≥ 4 points on the Peak Pruritus Numerical Rating Scale (PP-NRS), with scores ranging from 0 to 10 (higher scores indicating more severe itch) and an IGA response of 0 (clear) or 1 (almost clear) accompanied by a reduction from baseline to week 16 of ≥ 2 points. At week 16, higher proportions of patients in the nemolizumab group compared to those in the placebo group achieved an itch response (56.3% versus 20.9%; p<0.001) and an IGA response (37.7% versus 11%; p<0.001). Significant improvements in itch intensity and sleep disturbance, both secondary endpoints, were seen by week 4 with nemolizumab compared to placebo. The most common TEAEs with nemolizumab were headache and atopic dermatitis. Top-line results from the OLYMPIA 1 trial (NCT04501666) reported similar outcomes based on itch response and IGA response with nemolizumab compared to placebo (itch, 58.4% versus 16.7%, respectively [p<0.0001]; IGA, 26.3% versus 7.3%, respectively [p<0.0001]) at 16 weeks. In addition, interim data from the open-label, phase 3, long-term OLYMPIA-LTE trial (NCT04204616) demonstrated continuous improvement in skin lesions, itch, sleep disturbance, and QOL up to week 52 with nemolizumab. No new safety signals were reported at 52 weeks.
Nemolizumab was also evaluated in the identical ongoing, randomized, double-blind, phase 3 ARCADIA 1 (NCT03985943) and ARCADIA 2 (NCT03989349) trials for the treatment of moderate to severe atopic dermatitis. Combined, the trials enrolled over 1,700 patients ≥ 12 years of age who continued background topical steroid or topical calcineurin inhibitor therapy. After 16 weeks, 43.5% of patients in ARCADIA 1 and 42.1% in ARCADIA 2 who were treated with nemolizumab achieved the primary endpoint of 75% reduction in the Eczema Area and Severity Index (EASI) compared to 29% and 30.2%, respectively, of patients who received placebo (p<0.0001 and p=0.0011). In both studies, significant improvement in sleep disturbance (secondary endpoint) was also seen at 16 weeks with nemolizumab compared to placebo. Galderma has also initiated a long-term trial, ARCADIA-LTE, for nemolizumab for moderate to severe atopic dermatitis.
Dosage and administration
In the clinical trials, nemolizumab was self-administered SC at an initial 60 mg dose, followed by 30 mg or 60 mg, depending on baseline body weight, every 4 weeks.
PLACE IN THERAPY
Prurigo nodularis (PN) is a rare, chronic inflammatory skin condition characterized by raised, hyperkeratotic lesions on the arms, legs, and trunk. PN is often severely itchy and can have a marked negative effect on sleep and QOL. PN affects about 72 out of every 100,000 people in the U.S. People at increased risk for developing PN include patients ≥ 50 years of age, people who are Black, and those who have chronic conditions, such as atopic dermatitis, diabetes, ESRD, hepatitis C, untreated HIV, lymphoma, thyroid conditions, anxiety, or depression. PN treatments include topical emollients, menthol, pramoxine, capsaicin, corticosteroids, calcineurin inhibitors, and calcipotriol. Intradermal corticosteroids, cryosurgery, and phototherapy are useful in patient who fail topical agents. Systemic agents include antihistamines, as well as off-label use of antidepressants, gabapentin, methotrexate and thalidomide. In September 2022, the IL-4 receptor alpha antagonist dupilumab (Dupixent) became the first agent FDA-approved for PN in adults. In clinical trials, Dupixent led to reduction in itch (based on Worst Itch Numeric Rating Scale [WI-NRS] by ≥ 4 points from baseline) in 60% of patients and IGA of 0 or 1 in 48% of patients, compared to 18.4% of patients for each measure in the placebo group.
Atopic dermatitis (AD) is a common, chronic inflammatory skin condition characterized by persistent itch and recurrent skin lesions. It affects an estimated 31.6 million (10.1%) people in the U.S., including 9.6 million children and adolescents. Approximately 30% and 40% of cases in pediatrics and adults, respectively, are moderate to severe. Onset typically occurs before 6 years of age. While several systemic DMTs are available to treat moderate and severe AD, topical emollients, corticosteroids, and immunomodulators remain important options; however, long-term continuous use of topical steroids and immunomodulators is limited by TEAEs. The Janus kinase inhibitors abrocitinib (Cibinqo®; oral), upadacitinb (Rinvoq®; oral), and ruxolitinib (Opzelura®; topical), topical PDE5 inhibitor crisaborole (Eucrisa®), injectable IL‑13 inhibitor tralokinumab (Adbry®) and IL-4/IL-13 inhibitor dupilumab (Dupixent) are also indicated to treat AD.
Nemolizumab is a first-in-class monoclonal antibody that inhibits IL-31 and is designed to reduce burdensome itch related to chronic inflammatory skin conditions. If approved, it will be the second biologic agent approved for PN and will compete directly with Dupixent, a drug with efficacy and safety data across several immunologic conditions. Although there are no head-to-head trials, nemolizumab appears to demonstrate similar safety and efficacy to Dupixent in patients with PN, but may also gain approval in adolescents, an age group that Dupixent does not cover for PN. In addition, nemolizumab has been submitted to the FDA to treat atopic dermatitis in adults and adolescent patients. If approved, it may compete with Dupixent and Adbry in this space.
FDA APPROVAL TIMELINE
- Prurigo nodularis: August 14, 2024
FDA designations: Breakthrough Therapy, Priority Review
- Atopic dermatitis: December 14, 2024
FINANCIAL FORECAST (reported in millions)
The projected total U.S. sales for nemolizumab are not available.
Oncology – tarlatamab
tarlatamab IV
Manufacturer: Amgen
PROPOSED INDICATIONS
Advanced small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy
CLINICAL OVERVIEW
Mechanism of action
Tarlatamab is a bispecific T-cell engager (BiTE) that targets delta-like ligand 3 (DLL3) in neuroendocrine cancers, including SCLC.
Clinical trials
The open-label, phase 2 DeLLphi-301 trial (NCT05060016) evaluated tarlatamab in 220 patients with SCLC who had relapsed after, or was refractory to, one platinum-based treatment regimen and at least one other line of therapy. Eligible patients included those with asymptomatic, treated, stable brain metastases. Patients were randomized 1:1 to receive tarlatamab 10 mg or 100 mg doses. In the 10 mg group, at a median follow-up of 10.6 months, the ORR (primary endpoint) was 40%, with a complete response rate of 1%. In the 10 mg group, 59% and 25% of patients had a DOR ≥ 6 months and ≥ 9 months, respectively, and the PFS was 4.9 months. In the 100 mg group, at a median follow-up of 10.3 months, the ORR was 32%, with a complete response rate of 8%. Among patients who received 100 mg, 61% and 36% experienced a DOR ≥ 6 months and ≥ 9 months, respectively, and the PFS was 3.9 months. Most patients with an evaluable tumor-tissue sample (96% in each group) had DLL3 tumor expression; however, no difference in tumor reduction was seen based on DLL3 expression. The most common TEAEs reported were CRS and pyrexia. Grade 3 TEAEs occurred less often with the 10 mg dose (1%) versus the 100 mg dose (6%).
Dosage and administration
In the clinical trial, tarlatamab was administered IV over 60 minutes at a dose of 10 mg or 100 mg every 2 weeks.
PLACE IN THERAPY
Approximately 20% of all lung cancers are neuroendocrine tumors, of which nearly 14% are SCLC. Most cases of SCLC are associated with cigarette smoking. SCLC is an aggressive disease in which widespread metastases develop early in the disease course. The incidence of new cases of SCLC in the U.S. in 2023 was estimated at 33,000.
Systemic therapy is the SOC for patients with SCLC. A platinum agent + etoposide is recommended for all stages of the disease, with the addition of a PD-L1 blocker to treat extensive-stage disease, including cases in which brain metastases are present. Subsequent systemic therapies include platinum-based doublet, lurbinectedin, topotecan and irinotecan. In addition, concurrent radiation therapy may provide benefit at all stages of the disease as part of treatment and palliation; however, surgical resection is recommended only in a small proportion (5%) of patients with stage I-IIA (T1-2, N0, M0) SCLC.
Response rates to initial therapy are high, at about 70% to 90% for limited disease and approximately 60% for extensive disease. Unfortunately, most patients will relapse, and response to subsequent systemic therapy is poor and dependent on the time elapsed from initial therapy (response rate, ≤ 10% with interval ≤ 6 months and 25% with interval > 6 months).
DLL3 is highly expressed on the cell surface of neuroendocrine tumors and is present in the majority (≥ 85%) of patients with SCLC, making it a potential target in treating SCLC. Tarlatamab is a first-in-class agent that binds to both DLL3 on cancer cells and CD3 on T cells, leading to T cell-mediated lysis of the tumor cells. If approved, it will provide a new approach to combat SCLC, a disease with a large unmet need. It remains to be seen if tarlatamab will be used as second- or third-line systemic treatment. Most patients in the DeLLphi-301 clinical trial had ≥ 2 prior lines of therapy; however, the NCCN guidelines for SCLC do not distinguish between second- and third-line therapies, and instead offer recommendations based on primary and subsequent treatment options.
Amgen is also evaluating tarlatamab in phase 3 trials for SCLC, which include DeLLphi-304 comparing tarlatamab monotherapy with SOC chemotherapy (lurbinectedin, topotecan, amrubidin) for second-line treatment and DeLLphi-306 evaluating tarlatamab after chemoradiotherapy in earlier settings of SCLC.
FDA APPROVAL TIMELINE
June 12, 2024
FDA designations: Breakthrough Therapy, Orphan Drug, Priority Review, RTOR
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected annual U.S. sales | $21 | $69 | $128 | $205 | $274 |
Behavioral health – xanomeline-trospium (KarXT)
xanomeline-trospium (KarXT) oral
Manufacturer: Karuna
PROPOSED INDICATIONS
Schizophrenia
CLINICAL OVERVIEW
Mechanism of action
KarXT is a combination of xanomeline and trospium. Xanomeline is an antipsychotic agent with dual M1/M4 muscarinic acetylcholine receptor agonist activity and trospium is a muscarinic receptor antagonist that counteracts the peripheral muscarinic adverse effects of xanomeline.
Clinical trials
The randomized, double-blind, phase 3 EMERGENT-2 trial (NCT04659161) evaluated KarXT in 252 patients 18 to 65 years of age with schizophrenia who were hospitalized due to a recent worsening of psychosis (PANSS score ≥ 80, CGI-S ≥ 4). After a washout period of their background schizophrenia medications, patients were randomized 1:1 to KarXT or placebo. At week 5, patients treated with KarXT showed a statistically significant and clinically meaningful reduction in the PANSS total score (primary endpoint) compared to patients who were given placebo (mean change from baseline, -21.2 versus -11.6 points, respectively; difference, -9.6 points; p<0.0001). At week 5, KarXT also led to significant improvements from baseline in secondary endpoints compared to placebo including the PANSS positive subscale (-6.8 versus -3.9 points, respectively), PANSS negative subscale (-3.4 versus -1.6 points, respectively), PANSS Marder Factor negative score (-4.2 versus -2 points, respectively) and CGI-S (-1.2 versus -0.7 points, respectively) as well as the proportion of patients who experienced ≥ 30% reduction in PANSS total score (54.8% versus 28.3%, respectively) (p<0.05 for each endpoint). The most common TEAEs with KarXT (≥ 10%) were mild to moderate constipation, dyspepsia, headache, nausea, vomiting and hypertension. Discontinuation rates were similar between the two groups. KarXT was not associated with extrapyramidal or metabolic effects.
In the similarly designed randomized, double-blind, phase 3 EMERGENT-3 trial ((NCT04738123; n=256), KarXT also met its primary endpoint of reduction in PANSS total score at week 5 with an 8.4-point reduction in PANSS total score compared to placebo (p<0.0001). In addition, it demonstrated a clinically meaningful and statistically significant 3.5-point reduction in PANSS positive subscale compared to placebo (-7.1 versus -3.6 points, respectively; p<0.0001). While it showed reductions in the PANSS negative subscale, the change from baseline was not statistically significant at week 5; improvement in this subscale only achieved statistical significance at week 4 (p<0.05). The adverse event profile was generally similar to that reported in EMERGENT-2.
The PANSS total score ranges from 30 to 210 points. PANSS positive, negative and Marder Factor negative subscale scores each range from 7 to 49 points. A decrease in the PANSS score correlates with an improvement in schizophrenia symptoms. The CGI-S score ranges from 1 to 7, with 7 indicating the most severe illness.
Dosage and administration
In the clinical trials, KarXT was administered orally twice daily at xanomeline/trospium doses of 50 mg/20 mg on days 1 and 2, then 100 mg/20 mg on days 3 through 7. Beginning on day 8, KarXT dosing was flexible with an optional increase to 125 mg/30 mg and the option to return to 100 mg/20 mg based on tolerability.
PLACE IN THERAPY
Schizophrenia is a serious mental illness that affects 1% of the population. Schizophrenia manifests as positive symptoms (hallucination, delusion, and thought and movement disorder), negative symptoms (lack of emotion, pleasure, motivation and social interaction), and cognitive deficits (impaired executive function, attention and working memory). The onset of schizophrenia usually occurs between the late teens and the mid-30s. Schizophrenia can be debilitating, leading to non-fatal suicide attempts in approximately 20% to 40% of patients, and fatal attempts in approximately 4% to 5% of patients.
Psychosocial therapy, including cognitive-behavioral therapy, cognitive remediation, and social cognition training, is essential treatment for schizophrenia. Antipsychotics are the standard drugs used in patients with schizophrenia. They include first-generation antipsychotics (FGAs) (e.g., chlorpromazine, fluphenazine, haloperidol, loxapine, perphenazine, thiothixene, trifluoperazine) and second-generation antipsychotics (SGAs), also known as atypical antipsychotics (e.g., aripiprazole, asenapine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, risperidone, quetiapine, ziprasidone). Available antipsychotic agents have proven efficacy in treating positive symptoms of schizophrenia, but their efficacy for negative or cognitive symptoms is limited. FGAs work primarily by blocking dopamine (D2) receptors in the brain and are associated with extrapyramidal adverse effects, tardive dyskinesia, and hyperprolactinemia. SGAs block dopamine D2 receptors and serotonin 2A (5-HT2A) receptors and are generally more likely to cause weight gain and abnormal glucose and lipid levels. The approach to treatment of schizophrenia is largely based on trial and error due to patient variation in response and medication side effects. There is no preference between FGAs or SGAs, or between drugs within each group. However, clozapine is recommended in patients with treatment-resistant schizophrenia and in patients with a significant risk of suicide or aggressive behavior but is not recommended as first-line treatment due to serious side effects (e.g., agranulocytosis).
There are significant unmet needs in the treatment of schizophrenia, especially for the treatment of cognitive impairment and negative symptoms. In addition, side effect profiles contribute to a non-adherence rate estimated at 40% to 50%. Also, approximately 20% of patients will relapse after 1 year of being on an antipsychotic agent.
If approved, KarXT will herald in the first new mechanism of action to treat schizophrenia in several decades. Unlike FGAs and SGAs, it provides selective M1/M4 muscarinic receptor agonism through xanomeline without directly impacting dopamine D2 receptors and mitigates muscarinic TEAEs through the use of trospium. Phase 3 trials demonstrate that KarXT is effective for treating positive symptoms of schizophrenia in the short-term; however, the effects on negative symptoms and cognitive processes are inconclusive. Long-term efficacy and safety, particularly regarding tardive dyskinesia and metabolic effects, are uncertain. Due to the lack of long-term data, ICER rated the net health benefit of KarXT as promising, but inconclusive.
KarXT is also in a phase 3 trial as adjunctive therapy in schizophrenia (ARISE trial; non-hospitalized patients) and for the treatment of neuropsychiatric symptoms in Alzheimer’s disease (ADEPT program).
FDA APPROVAL TIMELINE
September 26, 2024
FINANCIAL FORECAST (reported in millions)
| Year | 2024 | 2025 | 2026 | 2027 | 2028 |
| Projected Yearly U.S. Sales | $43 | $307 | $700 | $1,085 | $1,371 |
Pipeline
Keep on your radar
Notable agents that are further from approval have been identified in this unique watch list. These are products with the potential for significant clinical and financial impact. Their development status is being tracked on the Quarterly Pipeline radar. These pipeline products, their respective class or proposed indication, as well as an estimated financial forecast for the year 2028, are displayed. The financials are projected total annual U.S. sales, reported in millions.
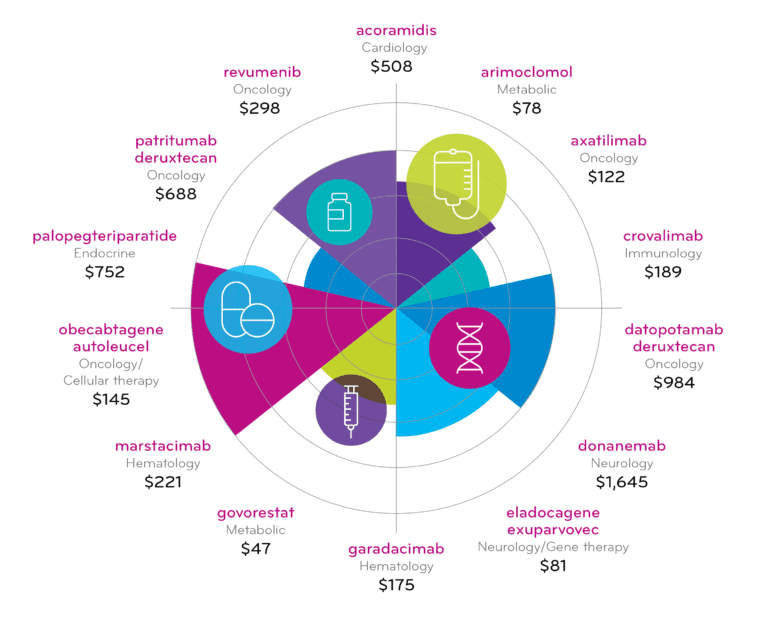
Table view
| Drug Generic Name | Therapeutic category | April 2024 Pipeline – Total US sales for 2028 (Dollars in millions) |
| acoramidis | Cardiology | $508 |
| arimoclomol | Metabolic | $78 |
| axatilimab | Oncology | $122 |
| crovalimab | Immunology | $189 |
| datopotamab deruxtecan | Oncology | $984 |
| donanemab | Neurology | $1,645 |
| eladocagene exuparvovec | Neurology/Gene Tx | $81 |
| garadacimab | Hematology | $175 |
| govorestat | Metabolic | $47 |
| marstacimab | Hematology | $221 |
| obecabtagene autoleucel | Oncology/Cellular therapy | $145 |
| palopegteriparatide | Endocrine | $752 |
| patritumab deruxtecan | Oncology | $688 |
| revumenib | Oncology | $298 |
Pipeline
Drug list
The pipeline drug list is an aerial outline of drugs with anticipated FDA approval through 2026. It is not intended to be a comprehensive inventory of all drugs in the pipeline; emphasis is placed on drugs in high-impact categories. Investigational drugs with a recent Complete Response Letter (CRL) are also reported.
Gene & cellular therapies
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| fidanacogene elaparvovec | Pfizer/Genentech | Hemophilia B (adults) | IV | BLA; Breakthrough Therapy; Orphan Drug; RMAT | Apr-Jun 2024 |
| prademagene zamikeracel | Abeona | Epidermolysis bullosa (recessive dystrophic) | Topical | BLA; Breakthrough Therapy; Orphan Drug; Priority Review; RMAT; RPD | 05/25/2024 |
| marnetegragene autotemcel | Rocket | Leukocyte adhesion deficiency-I | IV | BLA; Fast Track; Orphan Drug; Priority Review; RPD; RMAT | 06/29/2024 |
| afamitresgene autoleucel | Adaptimmune | Synovial sarcoma (advanced) | IV | BLA; Orphan Drug; Priority Review; RMAT | 08/04/2024 |
| obecabtagene autoleucel | Autolus | ALL (R/R, B cell) | IV | BLA; Orphan Drug; RMAT | 11/27/2024 |
| eladocagene exuparvovec | PTC | Aromatic L-amino acid decarboxylase (AADC) deficiency | Intracerebral | BLA; Orphan Drug; RPD | 03/19/2025 |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| lisocabtagene maraleucel (Breyanzi®) | Bristol-Myers Squibb | Follicular lymphoma (R/R) | IV | sBLA; Orphan Drug; Priority Review | 05/23/2024 |
| lisocabtagene maraleucel (Breyanzi) | Bristol-Myers Squibb | Mantle cell lymphoma (R/R, prior BTK inhibitor therapy) | IV | sBLA; Orphan Drug; Priority Review | 05/31/2024 |
| delandistrogene moxeparvovec (Elevidys) | Sarepta | DMD (confirmed mutation in the DMD gene; no age limit) | IV | sBLA; Fast Track; Orphan Drug; Priority Review; RPD | 06/21/2024 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| AAV8-ranibizumab (RGX-314) | Abbvie | DME; Wet AMD | Subretinal | BLA; Orphan Drug | TBD |
| aglatimagene besadenovec | Candel | Prostate cancer | Intratumorally | BLA; Fast Track | TBD |
| allogeneic adult stem cells (CAP-1002) | Nippon Shinyaku | DMD | IV | BLA; Orphan Drug; RMAT; RPD | TBD |
| autologous kidney cells (ReACT) | ProKidney | CKD | Hepatic injection | BLA; RMAT | TBD |
| botaretigene sparoparvovec | Janssen | Retinitis pigmentosa | Subretinal | BLA; Fast Track; Orphan Drug | TBD |
| dirloctocogene samoparvovec | Genentech | Hemophilia A | IV | BLA; Breakthrough, Orphan | TBD |
| giroctocogene fitelparvovec | Pfizer | Hemophilia A | IV | BLA; Fast Track; Orphan Drug; RMAT | TBD |
| RGX-121 | Regenxbio | Mucopolysaccharidosis II (MPS II; Hunter Syndrome) | CNS injection | BLA; Fast Track; Orphan Drug; RMAT; RPD | TBD |
| tabelecleucel | Pierre Fabre | Epstein-Barr virus-associated post-transplant lymphoproliferative disease | IV | BLA; Breakthrough Therapy; Orphan Drug | TBD |
Phase 3 supplemental drugs
None
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| fidanacogene elaparvovec | Pfizer/Genentech | Hemophilia B (adults) | IV | BLA; Breakthrough Therapy; Orphan Drug; RMAT | Apr-Jun 2024 |
| prademagene zamikeracel | Abeona | Epidermolysis bullosa (recessive dystrophic) | Topical | BLA; Breakthrough Therapy; Orphan Drug; Priority Review; RMAT; RPD | 05/25/2024 |
| marnetegragene autotemcel | Rocket | Leukocyte adhesion deficiency-I | IV | BLA; Fast Track; Orphan Drug; Priority Review; RPD; RMAT | 06/29/2024 |
| afamitresgene autoleucel | Adaptimmune | Synovial sarcoma (advanced) | IV | BLA; Orphan Drug; Priority Review; RMAT | 08/04/2024 |
| obecabtagene autoleucel | Autolus | ALL (R/R, B cell) | IV | BLA; Orphan Drug; RMAT | 11/27/2024 |
| eladocagene exuparvovec | PTC | Aromatic L-amino acid decarboxylase (AADC) deficiency | Intracerebral | BLA; Orphan Drug; RPD | 03/19/2025 |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| lisocabtagene maraleucel (Breyanzi®) | Bristol-Myers Squibb | Follicular lymphoma (R/R) | IV | sBLA; Orphan Drug; Priority Review | 05/23/2024 |
| lisocabtagene maraleucel (Breyanzi) | Bristol-Myers Squibb | Mantle cell lymphoma (R/R, prior BTK inhibitor therapy) | IV | sBLA; Orphan Drug; Priority Review | 05/31/2024 |
| delandistrogene moxeparvovec (Elevidys) | Sarepta | DMD (confirmed mutation in the DMD gene; no age limit) | IV | sBLA; Fast Track; Orphan Drug; Priority Review; RPD | 06/21/2024 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| AAV8-ranibizumab (RGX-314) | Abbvie | DME; Wet AMD | Subretinal | BLA; Orphan Drug | TBD |
| aglatimagene besadenovec | Candel | Prostate cancer | Intratumorally | BLA; Fast Track | TBD |
| allogeneic adult stem cells (CAP-1002) | Nippon Shinyaku | DMD | IV | BLA; Orphan Drug; RMAT; RPD | TBD |
| autologous kidney cells (ReACT) | ProKidney | CKD | Hepatic injection | BLA; RMAT | TBD |
| botaretigene sparoparvovec | Janssen | Retinitis pigmentosa | Subretinal | BLA; Fast Track; Orphan Drug | TBD |
| dirloctocogene samoparvovec | Genentech | Hemophilia A | IV | BLA; Breakthrough, Orphan | TBD |
| giroctocogene fitelparvovec | Pfizer | Hemophilia A | IV | BLA; Fast Track; Orphan Drug; RMAT | TBD |
| RGX-121 | Regenxbio | Mucopolysaccharidosis II (MPS II; Hunter Syndrome) | CNS injection | BLA; Fast Track; Orphan Drug; RMAT; RPD | TBD |
| tabelecleucel | Pierre Fabre | Epstein-Barr virus-associated post-transplant lymphoproliferative disease | IV | BLA; Breakthrough Therapy; Orphan Drug | TBD |
Phase 3 supplemental drugs
None
Biosimilars
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| insulin aspart (biosimilar to Novo Nordisk’s Novolog®) | Gan & Lee/Sandoz | T1DM; T2DM | SC | BLA | 4/14/2024 |
| ranibizumab (biosimilar to Genentech’s Lucentis®) | STADA Arzneimittel/Xbrane | Diabetic retinopathy; DME; Myopic choroidal neovascularization; Macular edema following RVO; Wet AMD | Intravitreal | BLA | 4/21/2024 |
| aflibercept (biosimilar to Regeneron’s Eylea®) | Amgen | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | May-Jul 2024 |
| rituximab (biosimilar to Genentech’s Rituxan®) | Dr. Reddy’s | CCL; Granulomatosis with polyangiitis/microscopic polyangiitis; NHL; Mature B-cell NHL/mature B-cell acute leukemia; Pemphigus vulgaris; RA | IV | BLA | 05/10/2024 |
| aflibercept (biosimilar to Regeneron’s Eylea) | Celltrion | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | 06/28/2024 |
| aflibercept (biosimilar to Regeneron’s Eylea) | Coherus | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | 06/28/2024 |
| ustekinumab (biosimilar to Janssen’s Stelara®) | Formycon | PSO; PsA; CD; UC | IV, SC | BLA | September 2024 |
| ustekinumab (biosimilar to Janssen’s Stelara) | Intas | PSO; PsA; CD; UC | SC | BLA | 11/04/2024 |
| ustekinumab (biosimilar to Janssen’s Stelara) | Biocon/Janssen | PSO; PsA; CD; UC | SC | BLA | 12/27/2024 |
| insulin aspart (biosimilar to Novo Nordisk’s Novolog) | Amphastar | T1DM; T2DM | SC | BLA | 01/10/2025 |
| tocilizumab (biosimilar to Genentech’s Actemra®) | Celltrion | RA; Giant cell arteritis; systemic sclerosis-associated interstitial lung disease; JIA (polyarticular, systemic); CRS; COVID-19 | IV, SC | BLA | 01/28/2025 |
| aflibercept (biosimilar to Regeneron’s Eylea) | Biocon/Janssen | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | Pending |
| eculizumab (biosimilar to Alexion’s Soliris®) | Amgen | PNH; Hemolytic uremic syndrome (atypical) | IV | BLA | Pending |
| insulin glargine (biosimilar to Sanofi’s Lantus®) | Gan & Lee/Sandoz | T1DM; T2DM | SC | BLA | Pending |
| insulin lispro (biosimilar to Eli Lilly’s Humalog®) | Gan & Lee/Sandoz | T1DM; T2DM | SC | BLA | Pending |
| pegfilgrastim (biosimilar to Amgen’s Neulasta®) | Lupin | Neutropenia/leukopenia | SC | BLA | Pending |
| trastuzumab (biosimilar to Genentech’s Herceptin®) | Henlius/Accord | Breast cancer; Gastric or gastroesophageal junction adenocarcinoma | IV | BLA | Pending |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| adalimumab-bwwd 40 mg/0.4 mL (Hadlima™) (biosimilar to Abbvie’s Humira®) | Organon | RA; AS; PSO; PsA; JIA; CD; UC; HS; Uveitis | SC | sBLA for interchangeability | 09/06/2024 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| aflibercept (biosimilar to Regeneron’s Eylea®) | Sandoz | DME; Wet AMD | Intravitreal | BLA | TBD |
| bevacizumab (biosimilar to Genentech’s Avastin®) | Essex | DME; Wet AMD | IV | BLA | TBD |
| insulin aspart (biosimilar to Novo Nordisk’s Novolog®) | Amphastar | T1DM | SC | BLA | TBD |
Phase 3 supplemental drugs
None
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| insulin aspart (biosimilar to Novo Nordisk’s Novolog®) | Gan & Lee/Sandoz | T1DM; T2DM | SC | BLA | 4/14/2024 |
| ranibizumab (biosimilar to Genentech’s Lucentis®) | STADA Arzneimittel/Xbrane | Diabetic retinopathy; DME; Myopic choroidal neovascularization; Macular edema following RVO; Wet AMD | Intravitreal | BLA | 4/21/2024 |
| aflibercept (biosimilar to Regeneron’s Eylea®) | Amgen | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | May-Jul 2024 |
| rituximab (biosimilar to Genentech’s Rituxan®) | Dr. Reddy’s | CCL; Granulomatosis with polyangiitis/microscopic polyangiitis; NHL; Mature B-cell NHL/mature B-cell acute leukemia; Pemphigus vulgaris; RA | IV | BLA | 05/10/2024 |
| aflibercept (biosimilar to Regeneron’s Eylea) | Celltrion | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | 06/28/2024 |
| aflibercept (biosimilar to Regeneron’s Eylea) | Coherus | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | 06/28/2024 |
| ustekinumab (biosimilar to Janssen’s Stelara®) | Formycon | PSO; PsA; CD; UC | IV, SC | BLA | September 2024 |
| ustekinumab (biosimilar to Janssen’s Stelara) | Intas | PSO; PsA; CD; UC | SC | BLA | 11/04/2024 |
| ustekinumab (biosimilar to Janssen’s Stelara) | Biocon/Janssen | PSO; PsA; CD; UC | SC | BLA | 12/27/2024 |
| insulin aspart (biosimilar to Novo Nordisk’s Novolog) | Amphastar | T1DM; T2DM | SC | BLA | 01/10/2025 |
| tocilizumab (biosimilar to Genentech’s Actemra®) | Celltrion | RA; Giant cell arteritis; systemic sclerosis-associated interstitial lung disease; JIA (polyarticular, systemic); CRS; COVID-19 | IV, SC | BLA | 01/28/2025 |
| aflibercept (biosimilar to Regeneron’s Eylea) | Biocon/Janssen | DME; Diabetic retinopathy; Macular edema following RVO; Wet AMD | Intravitreal | BLA | Pending |
| eculizumab (biosimilar to Alexion’s Soliris®) | Amgen | PNH; Hemolytic uremic syndrome (atypical) | IV | BLA | Pending |
| insulin glargine (biosimilar to Sanofi’s Lantus®) | Gan & Lee/Sandoz | T1DM; T2DM | SC | BLA | Pending |
| insulin lispro (biosimilar to Eli Lilly’s Humalog®) | Gan & Lee/Sandoz | T1DM; T2DM | SC | BLA | Pending |
| pegfilgrastim (biosimilar to Amgen’s Neulasta®) | Lupin | Neutropenia/leukopenia | SC | BLA | Pending |
| trastuzumab (biosimilar to Genentech’s Herceptin®) | Henlius/Accord | Breast cancer; Gastric or gastroesophageal junction adenocarcinoma | IV | BLA | Pending |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| adalimumab-bwwd 40 mg/0.4 mL (Hadlima™) (biosimilar to Abbvie’s Humira®) | Organon | RA; AS; PSO; PsA; JIA; CD; UC; HS; Uveitis | SC | sBLA for interchangeability | 09/06/2024 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| aflibercept (biosimilar to Regeneron’s Eylea®) | Sandoz | DME; Wet AMD | Intravitreal | BLA | TBD |
| bevacizumab (biosimilar to Genentech’s Avastin®) | Essex | DME; Wet AMD | IV | BLA | TBD |
| insulin aspart (biosimilar to Novo Nordisk’s Novolog®) | Amphastar | T1DM | SC | BLA | TBD |
Phase 3 supplemental drugs
None
Specialty
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| donanemab | Eli Lilly | Alzheimer’s disease (early, symptomatic) | IV | BLA; Breakthrough Therapy | Apr-Jun 2024 |
| nogapendekin alfa inbakicept | Immunitybio | Bladder cancer (BCG-unresponsive, non-muscle-invasive, carcinoma in situ [CIS], with or without Ta or T1 disease) | Intravesical | BLA; Breakthrough Therapy; Fast Track | 4/23/2024 |
| mavorixafor | X4 | Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome (ages ≥ 12 years) | Oral | NDA; Breakthrough Therapy; Fast Track; Orphan Drug; Priority Review; RPD | 04/30/2024 |
| tovorafenib | Day One | Glioma (relapsed/progressive, low-grade, monotherapy) | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review; RPD | 04/30/2024 |
| palopegteriparatide | Ascendis | Hypoparathyroidism | SC | NDA; Orphan Drug | 05/14/2024 |
| camrelizumab | Jiangsu Hengrui | HCC (unresectable, 1st-line, in combination with rivoceranib) | IV | BLA; Orphan Drug | 05/16/2024 |
| rivoceranib | Elevar | HCC (unresectable, 1st-line, in combination with camrelizumab) | Oral | NDA; Orphan Drug | 05/16/2024 |
| elafibranor | Genfit/Ipsen | Primary biliary cholangitis | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review | 06/10/2024 |
| tarlatamab | Amgen | SCLC (advanced) | IV | BLA; Breakthrough Therapy; Orphan Drug; Priority Review; RTOR | 06/12/2024 |
| imetelstat | Geron | Myelodysplastic syndrome (lower-risk, transfusion-dependent anemia, failed ESAs) | IV | NDA; Fast Track; Orphan Drug | 06/14/2024 |
| patritumab deruxtecan | Merck | NSCLC ( locally advanced or metastatic, EGFR-mutated, ≥ 2 prior systemic therapies) | IV | BLA; Breakthrough Therapy; Priority Review | 06/26/2024 |
| polyspecific immunoglobulin preparation | Biotest | Primary immunodeficiencies | IV | BLA | 06/29/2024 |
| SH-105 | Shorla | Breast cancer; Ovarian cancer | IV | NDA | 06/29/2024 |
| deuruxolitinib | Sun | Alopecia areata (moderate-severe) | Oral | NDA; Breakthrough Therapy; Fast Track | July 2024 |
| crovalimab | Genentech | PNH | IV, SC | BLA; Breakthrough Therapy; Orphan Drug | 07/27/2024 |
| dasatinib | Xspray | CML | Oral | 505(b)(2) NDA; Orphan Drug | 07/31/2024 |
| midomafetamine | Lykos | PTSD (in combination with trauma-focused psychotherapy) | Oral | NDA; Breakthrough Therapy; Priority Review | 08/11/2024 |
| denileukin diftitox | Citius | Cutaneous T cell lymphoma (R/R, ≥ 2nd-line) | IV | BLA; Orphan Drug | 08/13/2024 |
| nemolizumab | Galderma | Prurigo nodularis | SC | BLA; Breakthrough Therapy; Priority Review | 08/14/2024 |
| seladelpar | Cymabay | Primary biliary cholangitis (including pruritus, ursodeoxycholic acid inadequate response/intolerance) | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review | 08/14/2024 |
| vorasidenib | Servier | Glioma (diffuse, IDH-mutant ) | Oral | NDA; Breakthrough Therapy; Fast Track; Orphan Drug; Priority Review | 08/20/2024 |
| linvoseltamab | Regeneron | Multiple myeloma (R/R, 4th-line) | IV | BLA; Fast Track; Orphan Drug; Priority Review | 08/22/2024 |
| axatilimab | Syndax | Chronic GVHD (failure of ≥ 2 prior lines of systemic therapy, ages ≥ 6 years) | IV | BLA; Fast Track; Orphan Drug; Priority Review | 08/28/2024 |
| atezolizumab SC (Tecentriq®) | Genentech | Alveolar soft part sarcoma; HCC; Melanoma; NSCLC; SCLC; Urothelial cancer | SC | BLA | September 2024 |
| ocrelizumab SC (Ocrevus®) | Genentech | MS | SC | BLA | September 2024 |
| ACAM2000 | Emergent | Monkeypox (Mpox) | Scarification | BLA | 09/06/2024 |
| arimoclomol | Zevra | Niemann-Pick disease type C | Oral | NDA; Breakthrough Therapy; Fast Track; Orphan Drug; RPD | 09/21/2024 |
| N-acetyl-L-leucine | Intrabio | Niemann-Pick disease type C | Oral | NDA; Fast Track; Orphan Drug; Priority Review; RPD | 09/24/2024 |
| revumenib | Syndax/Abbvie | AML (R/R, KMT2A-rearranged) | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review; RTOR | 09/26/2024 |
| garadacimab | CSL | HAE | SC | BLA; Fast Track; Orphan Drug | Oct-Nov 2024 |
| marstacimab | Pfizer | Hemophilia A and B (without factor VIII or IX inhibitors) | IV, SC | BLA; Fast Track; Orphan Drug | Oct-Dec 2024 |
| paliperidone palmitate ER | Luye | Schizophrenia | IM | 505(b)(2) NDA | 10/09/2024 |
| octreotide | Novartis | Acromegaly | SC | NDA | 10/21/2024 |
| govorestat | Applied Therapeutics | Galactosemia | Oral | NDA; Fast Track; Orphan Drug; Priority Review; RPD | 11/28/2024 |
| acoramidis | Bridgebio | Transthyretin amyloid cardiomyopathy (ATTR-CM) | Oral | NDA | 11/29/2024 |
| zanidatamab | Jazz | Biliary tract cancer (unresectable, locally advanced or metastatic, HER2+, previously-treated) | IV | BLA; seeking Accelerated Approval; Breakthrough Therapy; Fast Track; Orphan Drug | Dec 2024-Apr 2025 |
| nemolizumab | Galderma | Atopic dermatitis (moderate-severe) | SC | BLA | 12/14/2024 |
| irinotecan liposome | CSPC | Pancreatic cancer | IV | 505(b)(2) NDA | 12/18/2024 |
| datopotamab deruxtecan | Daiichi Sankyo/AstraZeneca | NSCLC (locally advanced or metastatic, nonsquamous, ≥ 2-line) | IV | BLA | 12/20/2024 |
| glepaglutide | Zealand | Short bowel syndrome (dependent on parenteral support) | SC | NDA; Orphan Drug | 12/22/2024 |
| ensartinib | Xcovery | NSCLC (metastatic, ALK+) | Oral | NDA | 12/28/2024 |
| elamipretide | Stealth | Barth syndrome | SC | NDA; Fast Track; Orphan Drug; RPD | Jan-Feb 2025 |
| datopotamab deruxtecan | Daiichi Sankyo/AstraZeneca | Breast cancer (HR+/HER2-, IHC 0, IHC 1+ or IHC 2+/ISH-, unresectable or metastatic) | IV | BLA | 01/29/2025 |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| durvalumab (Imfinzi®) | AstraZeneca | NSCLC (neoadjuvant) | IV | sBLA; Breakthrough Therapy; Fast Track | Apr-Jun 2024 |
| valbenazine (Ingrezza®) oral granules | Neurocrine Biosciences | Huntington’s disease chorea; Tardive dyskinesia | Oral | sNDA; Orphan Drug | 04/30/2024 |
| rilpivirine (Edurant®) | Janssen | HIV-1 infection (in children weighting ≥ 10 kg) | Oral | sNDA | 05/28/2024 |
| alectinib (Alecensa®) | Genentech | NSCLC (ALK+, postoperative adjuvant) | Oral | sNDA; Priority Review | 05/30/2024 |
| vedolizumab (Entyvio®) | Takeda | CD (SC maintenance following IV induction) | SC | sBLA | Jun-Jul 2024 |
| sarilumab (Kevzara®) | Sanofi | JIA (polyarticular-course) | SC | sBLA | 06/10/2024 |
| repotrectinib (Augtyro™) | Bristol-Myers Squibb | Solid tumors (locally advanced or metastatic, NTRK gene fusion) | Oral | sNDA; Breakthrough Therapy; Fast Track; Priority Review | 06/15/2024 |
| adagrasib (Krazati®) | Bristol-Myers Squibb | CRC (KRASG12C mutated, locally advanced or metastatic, in combination with cetuximab) | Oral | sNDA; Breakthrough Therapy; Priority Review | 06/21/2024 |
| blinatumomab (Blincyto®) | Amgen | ALL (early-stage, CD19-positive B-cell precursor) | IV | sBLA; Breakthrough Therapy; Orphan Drug; Priority Review | 06/21/2024 |
| efgartigimod alfa/hyaluronidase-qvfc (Vyvgart® Hytrulo) | Argenx | Chronic inflammatory demyelinating polyneuropathy (CIDP) | SC | sBLA; Orphan Drug; Priority Review | 06/21/2024 |
| pembrolizumab (Keytruda®) | Merck | Endometrial carcinoma (primary advanced or recurrent, after chemotherapy) | IV | sBLA; Breakthrough Therapy; Priority Review | 06/21/2024 |
| pitolisant (Wakix®) | Harmony | Narcolepsy (excessive daytime sleepiness or cataplexy, ages 6 to 18 years) | Oral | sNDA; Breakthrough Therapy; Fast Track; Orphan Drug; Priority Review | 06/21/2024 |
| dupilumab (Dupixent®) | Sanofi/Regeneron | COPD (uncontrolled, add-on therapy) | SC | sBLA; Breakthrough Therapy; Priority Review | 06/27/2024 |
| epcoritamab-bysp (Epkinly™) | Genmab/Abbvie | Follicular lymphoma (R/R, ≥ 2 prior lines of systemic therapy) | SC | sBLA; Breakthrough Therapy; Orphan Drug; Priority Review | 06/28/2024 |
| risankizumab-rzaa (Skyrizi®) | Abbvie | UC | IV, SC | sBLA | 06/28/2024 |
| tislelizumab-jsgr (Tevimbra®) | Beigene | Esophageal squamous cell carcinoma (unresectable, recurrent, locally advanced, or metastatic, 1st-line) | IV | sBLA; Orphan Drug | July 2024 |
| benralizumab (Fasenra®) | AstraZeneca | Eosinophilic granulomatosis with polyangiitis | SC | sBLA; Orphan Drug | Jul-Dec 2024 |
| durvalumab (Imfinzi) | AstraZeneca | Endometrial cancer (1st-line, in combination with olaparib) | IV | sBLA | Jul-Dec 2024 |
| fam-trastuzumab deruxtecan-nxki (Enhertu®) | Daiichi Sankyo | Breast cancer (HER2+, 3rd-line) | IV | sBLA; Breakthrough Therapy; Fast Track | Jul-Dec 2024 |
| olaparib (Lynparza®) | AstraZeneca | Endometrial cancer (1st-line, in combination with durvalumab) | Oral | sNDA | Jul-Dec 2024 |
| amivantamab-vmjw (Rybrevant®) | Janssen | NSCLC (locally advanced or metastatic, EGFR exon 19 deletion or L858R substitution, disease progression on/after osimertinib); NSCLC (locally advanced or metastatic, EGFR exon 19 deletions or L858R substitution, 2nd-line, after disease progression on/after osimertinib, in combination with carboplatin-pemetrexed) | IV | sBLA; Breakthrough Therapy | 09/20/2024 |
| bimekizumab (Bimzelx®) | UCB | AS; PsA | SC | sBLA | October 2024 |
| iptacopan (Fabhalta®) | Novartis | IgA Nephropathy (Berger’s disease) | Oral | sNDA; seeking Accelerated Approval; Priority Review | October 2024 |
| nivolumab (Opdivo®) | Bristol-Myers Squibb | NSCLC (resectable stage IIA to IIIB, neoadjuvant with chemotherapy, and adjuvant) | IV | sBLA; Breakthrough Therapy | 10/08/2024 |
| amivantamab-vmjw (Rybrevant) | Janssen | NSCLC (locally advanced or metastatic, EGFR exon 19 deletion or L858R substitutionm in combination with lazertinib, 1st-line) | IV | sBLA | 10/21/2024 |
| avacincaptad pegol (Izervay™) | Astellas | Dry AMD-related geographic atrophy | Intravitreal | sNDA; Breakthrough Therapy; Fast Track | 11/19/2024 |
| daratumumab/hyaluronidase-fihj (Darzalex Faspro®) | Janssen | Multiple myeloma (newly diagnosed, in combination with bortezomib, lenalidomide and dexamethasone for induction and consolidation, and with lenalidomide for maintenance) | SC | sBLA | 11/28/2024 |
| bimekizumab (Bimzelx®) | UCB | HS (moderate to severe) | SC | sBLA | December 2024 |
| ribociclib (Kisqali®) | Novartis | Breast cancer (adjuvant) | Oral | sNDA; Breakthrough Therapy | December 2024 |
| tislelizumab-jsgr (Tevimbra®) | Beigene | Gastric or gastroesophageal junction adenocarcinoma (locally advanced unresectable or metastatic, in combination with fluoropyrimidine- and platinum-containing chemotherapy) | IV | sBLA | December 2024 |
| lecanemab-irmb (Leqembi®) | Eisai | Alzheimer’s disease (mild; once monthly dosing) | IV | sBLA; Breakthrough Therapy; Fast Track | January 2025 |
| guselkumab (Tremfya®) | Janssen | UC (moderate to severe) | SC | sBLA | 01/10/2025 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| aficamten | Cytokinetics | Hypertrophic cardiomyopathy | Oral | NDA; Breakthrough Therapy; Orphan Drug | TBD |
| anti-betv1 antibody (REGN-5713-5714-5715) | Regeneron | Birch allergy | SC | BLA | TBD |
| anti-BK polyomavirus | Memo | BK polyomavirus infection (renal transplant recipients) | IV | BLA; Fast Track | TBD |
| apraglutide | Ironwood | Short bowel syndrome | IV, SC | NDA; Orphan Drug | TBD |
| astegolimab | Genentech | COPD | IV | BLA | TBD |
| ataluren | PTC | DMD | Oral | NDA; Fast Track; Orphan Drug | TBD |
| atrasentan | Novartis | IgA nephropathy (Berger’s disease) | Oral | NDA | TBD |
| avutometinib | Verastem | Ovarian cancer | Oral | NDA; Breakthrough Therapy; Orphan Drug | TBD |
| cannabidiol gel | Harmony | Fragile X syndrome | Topical | NDA; Fast Track; Orphan Drug | TBD |
| cetuximab sarotalocan | Rakuten | SCCHN | IV | BLA; Fast Track | TBD |
| clesrovimab | Merck | RSV prevention | IM | BLA | TBD |
| cobolimab | GlaxoSmithKline | NSCLC | IV | BLA | TBD |
| crinecerfont | Neurocrine | Congenital adrenal hyperplasia | Oral | NDA; Breakthrough Therapy; Orphan Drug | TBD |
| crovalimab | Genentech | Hemolytic uremic syndrome | IV, SC | BLA | TBD |
| CTX-009 | Compass | Biliary tract cancer | IV | BLA | TBD |
| defactinib | Verastem | Ovarian cancer | Oral | NDA; Orphan Drug | TBD |
| deoxythymidine/deoxycytidine | UCB | Thymidine kinase 2 (TK2) deficiency | Oral | BLA; Breakthrough Therapy; Orphan Drug | TBD |
| depemokimab | GlaxoSmithKline | Asthma; Chronic rhinosinusitis | SC | BLA | TBD |
| dersimelagon | Mitsubishi Tanabe | Porphyria | Oral | NDA; Fast Track; Orphan Drug | TBD |
| dinutuximab beta | EUSA | Neuroendocrine tumors | IV | BLA; Orphan Drug | TBD |
| donidalorsen | Ionis | HAE | SC | NDA; Orphan Drug | TBD |
| efruxifermin | Akero | NASH | SC | BLA; Breakthrough Therapy; Fast Track | TBD |
| etavopivat | Novo Nordisk | SCD | Oral | NDA; Fast Track; Orphan Drug; RPD | TBD |
| fenebrutinib | Genentech | MS | Oral | NDA | TBD |
| fianlimab | Regeneron | Melanoma; NSCLC | IV | BLA; Fast Track | TBD |
| fitusiran | Sanofi | Hemophilia A and B | SC | NDA; Fast Track; Orphan Drug | TBD |
| garetosmab | Regeneron | Fibrodysplasia ossificans progressiva | IV | BLA; Fast Track; Orphan Drug | TBD |
| giredestrant | Genentech | Breast cancer (HR+/HER2-) | Oral | NDA; Fast Track | TBD |
| GBT601 | Pfizer | SCD | Oral | NDA; Orphan Drug | TBD |
| gold nanocrystal | Clene | ALS | Oral | NDA; Orphan Drug | TBD |
| ianalumab | Novartis | Autoimmune hemolytic anemia; Sjogren’s syndrome | SC | BLA | TBD |
| imlifidase | Sarepta | Kidney transplant rejection | IV | BLA; Fast Track; Orphan Drug | TBD |
| imsidolimab | Anaptysbio | Generalized pustular psoriasis | IV, SC | BLA; Orphan Drug | TBD |
| inavolisib | Genentech | Breast cancer (HR+/HER2-, 1st-line) | Oral | NDA | TBD |
| IONIS-FB-LRx | Ionis/Roche | IgA nephropathy (Berger’s disease) | SC | NDA | TBD |
| itepekimab | Regeneron | COPD | SC | BLA; Fast Track | TBD |
| JDQ-443 | Novartis | NSCLC | Oral | NDA | TBD |
| ketamine | Hope | Bipolar disorder | IV | NDA; Fast Track | TBD |
| latozinemab | Aldeyra | Frontotemporal dementia | IV | BLA; Breakthrough Therapy; Fast Track; Orphan Drug | TBD |
| lazertinib | Genosco/Janssen | NSCLC | Oral | NDA | TBD |
| lerodalcibep | LIB | Dyslipidemia/hypercholesterolemia; Heterozygous familial hypercholesterolemia (HeFH); Homozygous familial hypercholesterolemia (HoFH) | SC | BLA | TBD |
| leukocyte interleukin | Cel-Sci | SCCHN | SC | BLA; Orphan Drug | TBD |
| ligelizumab | Novartis | Food allergies | SC | BLA | TBD |
| linerixibat | GlaxoSmithKline | Primary biliary cholangitis pruritus | Oral | NDA; Orphan Drug | TBD |
| molgramostim | Savara | Pulmonary alveolar proteinosis | Inhaled | BLA; Breakthrough Therapy; Fast Track; Orphan Drug | TBD |
| navepegritide | Ascendis | Achondroplasia | SC | NDA; Orphan Drug | TBD |
| navitoclax | Abbvie | Myelofibrosis | Oral | NDA; Orphan Drug | TBD |
| nipocalimab | Janssen | Autoimmune hemolytic anemia; Myasthenia gravis | IV | BLA; Fast Track; Orphan Drug | TBD |
| obicetrapib | NewAmsterdam | Dyslipidemia/hypercholesterolemia | Oral | NDA | TBD |
| olezarsen | Akcea | Dyslipidemia/hypercholesterolemia; Familial chylomicronemia syndrome | SC | NDA; Fast Track; Orphan Drug | TBD |
| pabinafusp alfa | JCR | Mucopolysaccharidosis II (MPS II; Hunter Syndrome) | IV | BLA; Orphan Drug | TBD |
| paltusotine | Crinetics | Acromegaly | Oral | NDA; Orphan Drug | TBD |
| pegadricase | Swedish Orphan Biovitrum | Gout | IV | BLA | TBD |
| pegzilarginase | Immedica | Arginase 1 deficiency | IV | BLA; Breakthrough Therapy; Fast Track; Orphan Drug; RPD | TBD |
| pelabresib | Morphosys | Myelofibrosis | Oral | NDA; Fast Track; Orphan Drug | TBD |
| pelacarsen | Novartis | Dyslipidemia/hypercholesterolemia | SC | NDA; Fast Track | TBD |
| piclidenoson | Can-Fite | PSO | Oral | NDA | TBD |
| plozasiran | Arrowhead | Familial chylomicronemia syndrome | SC | NDA; Fast Track; Orphan Drug | TBD |
| ralinepag | United Therapeutics | PAH | Oral | NDA; Orphan Drug | TBD |
| relacorilant | Corcept | Cushing’s syndrome | Oral | NDA; Orphan Drug | TBD |
| remibrutinib | Novartis | MS; Urticaria | Oral | NDA | TBD |
| resiniferatoxin | Grunenthal | Osteoarthritis pain (knee) | Intra-articular | NDA; Breakthrough Therapy | TBD |
| rilzabrutinib | Sanofi | ITP | Oral | NDA; Fast Track; Orphan Drug | TBD |
| rusfertide | Takeda | Polycythemia vera | SC | NDA; Fast Track; Orphan Drug | TBD |
| sebetralstat | Kalvista | HAE | Oral | NDA; Fast Track; Orphan Drug | TBD |
| sepiapterin | PTC | Phenylketonuria (PKU) | Oral | NDA; Orphan Drug | TBD |
| serplulimab | Henlius | SCLC | IV | BLA; Orphan Drug | TBD |
| soticlestat | Takeda | Dravet syndrome; Lennox-Gastaut syndrome | Oral | NDA; Orphan Drug | TBD |
| TAK-279 | Takeda | PSO | Oral | NDA | TBD |
| tamibarotene | Syros | Myelodysplastic syndrome | Oral | NDA; Fast Track; Orphan Drug | TBD |
| telisotuzumab vedotin | Achieve Life Sciences | NSCLC | IV | BLA; Breakthrough Therapy | TBD |
| tiragolumab | Genentech | Esophageal cancer; NSCLC | IV | BLA; Breakthrough Therapy; Orphan Drug | TBD |
| tolebrutinib | Sanofi | MS | Oral | NDA | TBD |
| tiratricol | Rare Thyroid Therapeutics | Resistance to thyroid hormone type beta (RTH-b) | Oral | NDA; Fast Track; Orphan Drug; RPD | TBD |
| vanzacaftor/tezacaftor/deutivacaftor | Vertex | CF | Oral | NDA; Orphan Drug | TBD |
| vatiquinone | PTC | Friedreich’s ataxia | Oral | NDA; Fast Track; Orphan Drug | TBD |
| venglustat | Sanofi | GM2 gangliosidoses (Tay-Sachs disease, Sandhoff disease) | Oral | NDA; Orphan Drug | TBD |
| vimseltinib | Deciphera | Pigmented villonodular synovitis | Oral | NDA; Fast Track | TBD |
| zanidatamab | Jazz | Gastric cancer | IV | BLA; Fast Track; Orphan Drug | TBD |
| zatolmilast | Tetra | Fragile X syndrome | Oral | NDA; Orphan Drug; RPD | TBD |
Phase 3 supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| alpelisib (Piqray®) | Novartis | Breast cancer (HER2+) | Oral | sNDA | TBD |
| atezolizumab (Tecentriq®) | Genentech | Breast cancer (HER2+); Breast cancer (triple-negative) | IV | sBLA | TBD |
| brolucizumab-dbll (Beovu®) | Novartis | Diabetic retinopathy | Intravitreal | sBLA | TBD |
| canakinumab (Ilaris®) | Novartis | NSCLC (adjuvant) | SC | sBLA | TBD |
| cabozantinib (Cabometyx®/Cometriq®) | Exelixis | Prostate cancer | Oral | sNDA | TBD |
| cemiplimab-rwlc (Libtayo®) | Regeneron | Melanoma | IV | sBLA; Fast Track | TBD |
| dupilumab (Dupixent®) | Sanofi | Bullous pemphigoid | SC | sBLA; Orphan Drug | TBD |
| durvalumab (Imfinzi®) | AstraZeneca | Bladder cancer (1st-line) | IV | sBLA; Breakthrough Therapy | TBD |
| eplontersen (Wainua™) | Ionis | Transthyretin amyloid cardiomyopathy | SC | sNDA; Fast Track; Orphan Drug | TBD |
| ganaxolone (Ztalmy®) | Marinus | Status epilepticus (refractory); Tuberous sclerosis complex | IV, Oral | sNDA; Orphan Drug | TBD |
| iptacopan (Fabhalta®) | Novartis | C3 glomerulopathy; Hemolytic uremic syndrome; IgA nephropathy (Berger’s disease) | Oral | sNDA; Breakthrough Therapy; Orphan Drug; RPD | TBD |
| mepolizumab (Nucala®) | GlaxoSmithKline | COPD | IV, SC | sBLA | TBD |
| mirikizumab-mrkz (Omvoh™) | Eli Lilly | CD | IV, SC | sBLA | TBD |
| mitapivat (Pyrukynd®) | Agios | SCD; Thalassemia | Oral | sNDA; Orphan Drug | TBD |
| mosunetuzumab-axgb (Lunsumio®) | Genentech | DLBCL | SC | sBLA | TBD |
| obinutuzumab (Gazyva®) | Genentech | Lupus nephritis; Membranous nephropathy; SLE | IV | sBLA; Breakthrough Therapy | TBD |
| pozelimab (Veopoz™) | Regeneron | PNH | IV, SC | sBLA; Orphan Drug | TBD |
| ranibizumab port delivery system (Susvimo®) | Genentech | DME | Intravitreal implant | sBLA | TBD |
| satralizumab-mwge (Enspryng®) | Genentech | Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) | SC | sBLA; Orphan Drug | TBD |
| secukinumab (Cosentyx®) | Novartis | Giant cell arteritis; Lupus nephritis | SC | sBLA | TBD |
| sotorasib (Lumakras®) | Amgen | CRC | Oral | sNDA; Orphan Drug | TBD |
| venetoclax (Venclexta®) | Abbvie/Genenetech | Myelodysplastic syndrome; Multiple myeloma | Oral | sNDA; Breakthrough Therapy; Orphan Drug | TBD |
| vutrisiran (Amvuttra®) | Alnylam | Transthyretin amyloid cardiomyopathy | SC | sNDA; Orphan Drug | TBD |
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| donanemab | Eli Lilly | Alzheimer’s disease (early, symptomatic) | IV | BLA; Breakthrough Therapy | Apr-Jun 2024 |
| nogapendekin alfa inbakicept | Immunitybio | Bladder cancer (BCG-unresponsive, non-muscle-invasive, carcinoma in situ [CIS], with or without Ta or T1 disease) | Intravesical | BLA; Breakthrough Therapy; Fast Track | 4/23/2024 |
| mavorixafor | X4 | Warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome (ages ≥ 12 years) | Oral | NDA; Breakthrough Therapy; Fast Track; Orphan Drug; Priority Review; RPD | 04/30/2024 |
| tovorafenib | Day One | Glioma (relapsed/progressive, low-grade, monotherapy) | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review; RPD | 04/30/2024 |
| palopegteriparatide | Ascendis | Hypoparathyroidism | SC | NDA; Orphan Drug | 05/14/2024 |
| camrelizumab | Jiangsu Hengrui | HCC (unresectable, 1st-line, in combination with rivoceranib) | IV | BLA; Orphan Drug | 05/16/2024 |
| rivoceranib | Elevar | HCC (unresectable, 1st-line, in combination with camrelizumab) | Oral | NDA; Orphan Drug | 05/16/2024 |
| elafibranor | Genfit/Ipsen | Primary biliary cholangitis | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review | 06/10/2024 |
| tarlatamab | Amgen | SCLC (advanced) | IV | BLA; Breakthrough Therapy; Orphan Drug; Priority Review; RTOR | 06/12/2024 |
| imetelstat | Geron | Myelodysplastic syndrome (lower-risk, transfusion-dependent anemia, failed ESAs) | IV | NDA; Fast Track; Orphan Drug | 06/14/2024 |
| patritumab deruxtecan | Merck | NSCLC ( locally advanced or metastatic, EGFR-mutated, ≥ 2 prior systemic therapies) | IV | BLA; Breakthrough Therapy; Priority Review | 06/26/2024 |
| polyspecific immunoglobulin preparation | Biotest | Primary immunodeficiencies | IV | BLA | 06/29/2024 |
| SH-105 | Shorla | Breast cancer; Ovarian cancer | IV | NDA | 06/29/2024 |
| deuruxolitinib | Sun | Alopecia areata (moderate-severe) | Oral | NDA; Breakthrough Therapy; Fast Track | July 2024 |
| crovalimab | Genentech | PNH | IV, SC | BLA; Breakthrough Therapy; Orphan Drug | 07/27/2024 |
| dasatinib | Xspray | CML | Oral | 505(b)(2) NDA; Orphan Drug | 07/31/2024 |
| midomafetamine | Lykos | PTSD (in combination with trauma-focused psychotherapy) | Oral | NDA; Breakthrough Therapy; Priority Review | 08/11/2024 |
| denileukin diftitox | Citius | Cutaneous T cell lymphoma (R/R, ≥ 2nd-line) | IV | BLA; Orphan Drug | 08/13/2024 |
| nemolizumab | Galderma | Prurigo nodularis | SC | BLA; Breakthrough Therapy; Priority Review | 08/14/2024 |
| seladelpar | Cymabay | Primary biliary cholangitis (including pruritus, ursodeoxycholic acid inadequate response/intolerance) | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review | 08/14/2024 |
| vorasidenib | Servier | Glioma (diffuse, IDH-mutant ) | Oral | NDA; Breakthrough Therapy; Fast Track; Orphan Drug; Priority Review | 08/20/2024 |
| linvoseltamab | Regeneron | Multiple myeloma (R/R, 4th-line) | IV | BLA; Fast Track; Orphan Drug; Priority Review | 08/22/2024 |
| axatilimab | Syndax | Chronic GVHD (failure of ≥ 2 prior lines of systemic therapy, ages ≥ 6 years) | IV | BLA; Fast Track; Orphan Drug; Priority Review | 08/28/2024 |
| atezolizumab SC (Tecentriq®) | Genentech | Alveolar soft part sarcoma; HCC; Melanoma; NSCLC; SCLC; Urothelial cancer | SC | BLA | September 2024 |
| ocrelizumab SC (Ocrevus®) | Genentech | MS | SC | BLA | September 2024 |
| ACAM2000 | Emergent | Monkeypox (Mpox) | Scarification | BLA | 09/06/2024 |
| arimoclomol | Zevra | Niemann-Pick disease type C | Oral | NDA; Breakthrough Therapy; Fast Track; Orphan Drug; RPD | 09/21/2024 |
| N-acetyl-L-leucine | Intrabio | Niemann-Pick disease type C | Oral | NDA; Fast Track; Orphan Drug; Priority Review; RPD | 09/24/2024 |
| revumenib | Syndax/Abbvie | AML (R/R, KMT2A-rearranged) | Oral | NDA; Breakthrough Therapy; Orphan Drug; Priority Review; RTOR | 09/26/2024 |
| garadacimab | CSL | HAE | SC | BLA; Fast Track; Orphan Drug | Oct-Nov 2024 |
| marstacimab | Pfizer | Hemophilia A and B (without factor VIII or IX inhibitors) | IV, SC | BLA; Fast Track; Orphan Drug | Oct-Dec 2024 |
| paliperidone palmitate ER | Luye | Schizophrenia | IM | 505(b)(2) NDA | 10/09/2024 |
| octreotide | Novartis | Acromegaly | SC | NDA | 10/21/2024 |
| govorestat | Applied Therapeutics | Galactosemia | Oral | NDA; Fast Track; Orphan Drug; Priority Review; RPD | 11/28/2024 |
| acoramidis | Bridgebio | Transthyretin amyloid cardiomyopathy (ATTR-CM) | Oral | NDA | 11/29/2024 |
| zanidatamab | Jazz | Biliary tract cancer (unresectable, locally advanced or metastatic, HER2+, previously-treated) | IV | BLA; seeking Accelerated Approval; Breakthrough Therapy; Fast Track; Orphan Drug | Dec 2024-Apr 2025 |
| nemolizumab | Galderma | Atopic dermatitis (moderate-severe) | SC | BLA | 12/14/2024 |
| irinotecan liposome | CSPC | Pancreatic cancer | IV | 505(b)(2) NDA | 12/18/2024 |
| datopotamab deruxtecan | Daiichi Sankyo/AstraZeneca | NSCLC (locally advanced or metastatic, nonsquamous, ≥ 2-line) | IV | BLA | 12/20/2024 |
| glepaglutide | Zealand | Short bowel syndrome (dependent on parenteral support) | SC | NDA; Orphan Drug | 12/22/2024 |
| ensartinib | Xcovery | NSCLC (metastatic, ALK+) | Oral | NDA | 12/28/2024 |
| elamipretide | Stealth | Barth syndrome | SC | NDA; Fast Track; Orphan Drug; RPD | Jan-Feb 2025 |
| datopotamab deruxtecan | Daiichi Sankyo/AstraZeneca | Breast cancer (HR+/HER2-, IHC 0, IHC 1+ or IHC 2+/ISH-, unresectable or metastatic) | IV | BLA | 01/29/2025 |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| durvalumab (Imfinzi®) | AstraZeneca | NSCLC (neoadjuvant) | IV | sBLA; Breakthrough Therapy; Fast Track | Apr-Jun 2024 |
| valbenazine (Ingrezza®) oral granules | Neurocrine Biosciences | Huntington’s disease chorea; Tardive dyskinesia | Oral | sNDA; Orphan Drug | 04/30/2024 |
| rilpivirine (Edurant®) | Janssen | HIV-1 infection (in children weighting ≥ 10 kg) | Oral | sNDA | 05/28/2024 |
| alectinib (Alecensa®) | Genentech | NSCLC (ALK+, postoperative adjuvant) | Oral | sNDA; Priority Review | 05/30/2024 |
| vedolizumab (Entyvio®) | Takeda | CD (SC maintenance following IV induction) | SC | sBLA | Jun-Jul 2024 |
| sarilumab (Kevzara®) | Sanofi | JIA (polyarticular-course) | SC | sBLA | 06/10/2024 |
| repotrectinib (Augtyro™) | Bristol-Myers Squibb | Solid tumors (locally advanced or metastatic, NTRK gene fusion) | Oral | sNDA; Breakthrough Therapy; Fast Track; Priority Review | 06/15/2024 |
| adagrasib (Krazati®) | Bristol-Myers Squibb | CRC (KRASG12C mutated, locally advanced or metastatic, in combination with cetuximab) | Oral | sNDA; Breakthrough Therapy; Priority Review | 06/21/2024 |
| blinatumomab (Blincyto®) | Amgen | ALL (early-stage, CD19-positive B-cell precursor) | IV | sBLA; Breakthrough Therapy; Orphan Drug; Priority Review | 06/21/2024 |
| efgartigimod alfa/hyaluronidase-qvfc (Vyvgart® Hytrulo) | Argenx | Chronic inflammatory demyelinating polyneuropathy (CIDP) | SC | sBLA; Orphan Drug; Priority Review | 06/21/2024 |
| pembrolizumab (Keytruda®) | Merck | Endometrial carcinoma (primary advanced or recurrent, after chemotherapy) | IV | sBLA; Breakthrough Therapy; Priority Review | 06/21/2024 |
| pitolisant (Wakix®) | Harmony | Narcolepsy (excessive daytime sleepiness or cataplexy, ages 6 to 18 years) | Oral | sNDA; Breakthrough Therapy; Fast Track; Orphan Drug; Priority Review | 06/21/2024 |
| dupilumab (Dupixent®) | Sanofi/Regeneron | COPD (uncontrolled, add-on therapy) | SC | sBLA; Breakthrough Therapy; Priority Review | 06/27/2024 |
| epcoritamab-bysp (Epkinly™) | Genmab/Abbvie | Follicular lymphoma (R/R, ≥ 2 prior lines of systemic therapy) | SC | sBLA; Breakthrough Therapy; Orphan Drug; Priority Review | 06/28/2024 |
| risankizumab-rzaa (Skyrizi®) | Abbvie | UC | IV, SC | sBLA | 06/28/2024 |
| tislelizumab-jsgr (Tevimbra®) | Beigene | Esophageal squamous cell carcinoma (unresectable, recurrent, locally advanced, or metastatic, 1st-line) | IV | sBLA; Orphan Drug | July 2024 |
| benralizumab (Fasenra®) | AstraZeneca | Eosinophilic granulomatosis with polyangiitis | SC | sBLA; Orphan Drug | Jul-Dec 2024 |
| durvalumab (Imfinzi) | AstraZeneca | Endometrial cancer (1st-line, in combination with olaparib) | IV | sBLA | Jul-Dec 2024 |
| fam-trastuzumab deruxtecan-nxki (Enhertu®) | Daiichi Sankyo | Breast cancer (HER2+, 3rd-line) | IV | sBLA; Breakthrough Therapy; Fast Track | Jul-Dec 2024 |
| olaparib (Lynparza®) | AstraZeneca | Endometrial cancer (1st-line, in combination with durvalumab) | Oral | sNDA | Jul-Dec 2024 |
| amivantamab-vmjw (Rybrevant®) | Janssen | NSCLC (locally advanced or metastatic, EGFR exon 19 deletion or L858R substitution, disease progression on/after osimertinib); NSCLC (locally advanced or metastatic, EGFR exon 19 deletions or L858R substitution, 2nd-line, after disease progression on/after osimertinib, in combination with carboplatin-pemetrexed) | IV | sBLA; Breakthrough Therapy | 09/20/2024 |
| bimekizumab (Bimzelx®) | UCB | AS; PsA | SC | sBLA | October 2024 |
| iptacopan (Fabhalta®) | Novartis | IgA Nephropathy (Berger’s disease) | Oral | sNDA; seeking Accelerated Approval; Priority Review | October 2024 |
| nivolumab (Opdivo®) | Bristol-Myers Squibb | NSCLC (resectable stage IIA to IIIB, neoadjuvant with chemotherapy, and adjuvant) | IV | sBLA; Breakthrough Therapy | 10/08/2024 |
| amivantamab-vmjw (Rybrevant) | Janssen | NSCLC (locally advanced or metastatic, EGFR exon 19 deletion or L858R substitutionm in combination with lazertinib, 1st-line) | IV | sBLA | 10/21/2024 |
| avacincaptad pegol (Izervay™) | Astellas | Dry AMD-related geographic atrophy | Intravitreal | sNDA; Breakthrough Therapy; Fast Track | 11/19/2024 |
| daratumumab/hyaluronidase-fihj (Darzalex Faspro®) | Janssen | Multiple myeloma (newly diagnosed, in combination with bortezomib, lenalidomide and dexamethasone for induction and consolidation, and with lenalidomide for maintenance) | SC | sBLA | 11/28/2024 |
| bimekizumab (Bimzelx®) | UCB | HS (moderate to severe) | SC | sBLA | December 2024 |
| ribociclib (Kisqali®) | Novartis | Breast cancer (adjuvant) | Oral | sNDA; Breakthrough Therapy | December 2024 |
| tislelizumab-jsgr (Tevimbra®) | Beigene | Gastric or gastroesophageal junction adenocarcinoma (locally advanced unresectable or metastatic, in combination with fluoropyrimidine- and platinum-containing chemotherapy) | IV | sBLA | December 2024 |
| lecanemab-irmb (Leqembi®) | Eisai | Alzheimer’s disease (mild; once monthly dosing) | IV | sBLA; Breakthrough Therapy; Fast Track | January 2025 |
| guselkumab (Tremfya®) | Janssen | UC (moderate to severe) | SC | sBLA | 01/10/2025 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| aficamten | Cytokinetics | Hypertrophic cardiomyopathy | Oral | NDA; Breakthrough Therapy; Orphan Drug | TBD |
| anti-betv1 antibody (REGN-5713-5714-5715) | Regeneron | Birch allergy | SC | BLA | TBD |
| anti-BK polyomavirus | Memo | BK polyomavirus infection (renal transplant recipients) | IV | BLA; Fast Track | TBD |
| apraglutide | Ironwood | Short bowel syndrome | IV, SC | NDA; Orphan Drug | TBD |
| astegolimab | Genentech | COPD | IV | BLA | TBD |
| ataluren | PTC | DMD | Oral | NDA; Fast Track; Orphan Drug | TBD |
| atrasentan | Novartis | IgA nephropathy (Berger’s disease) | Oral | NDA | TBD |
| avutometinib | Verastem | Ovarian cancer | Oral | NDA; Breakthrough Therapy; Orphan Drug | TBD |
| cannabidiol gel | Harmony | Fragile X syndrome | Topical | NDA; Fast Track; Orphan Drug | TBD |
| cetuximab sarotalocan | Rakuten | SCCHN | IV | BLA; Fast Track | TBD |
| clesrovimab | Merck | RSV prevention | IM | BLA | TBD |
| cobolimab | GlaxoSmithKline | NSCLC | IV | BLA | TBD |
| crinecerfont | Neurocrine | Congenital adrenal hyperplasia | Oral | NDA; Breakthrough Therapy; Orphan Drug | TBD |
| crovalimab | Genentech | Hemolytic uremic syndrome | IV, SC | BLA | TBD |
| CTX-009 | Compass | Biliary tract cancer | IV | BLA | TBD |
| defactinib | Verastem | Ovarian cancer | Oral | NDA; Orphan Drug | TBD |
| deoxythymidine/deoxycytidine | UCB | Thymidine kinase 2 (TK2) deficiency | Oral | BLA; Breakthrough Therapy; Orphan Drug | TBD |
| depemokimab | GlaxoSmithKline | Asthma; Chronic rhinosinusitis | SC | BLA | TBD |
| dersimelagon | Mitsubishi Tanabe | Porphyria | Oral | NDA; Fast Track; Orphan Drug | TBD |
| dinutuximab beta | EUSA | Neuroendocrine tumors | IV | BLA; Orphan Drug | TBD |
| donidalorsen | Ionis | HAE | SC | NDA; Orphan Drug | TBD |
| efruxifermin | Akero | NASH | SC | BLA; Breakthrough Therapy; Fast Track | TBD |
| etavopivat | Novo Nordisk | SCD | Oral | NDA; Fast Track; Orphan Drug; RPD | TBD |
| fenebrutinib | Genentech | MS | Oral | NDA | TBD |
| fianlimab | Regeneron | Melanoma; NSCLC | IV | BLA; Fast Track | TBD |
| fitusiran | Sanofi | Hemophilia A and B | SC | NDA; Fast Track; Orphan Drug | TBD |
| garetosmab | Regeneron | Fibrodysplasia ossificans progressiva | IV | BLA; Fast Track; Orphan Drug | TBD |
| giredestrant | Genentech | Breast cancer (HR+/HER2-) | Oral | NDA; Fast Track | TBD |
| GBT601 | Pfizer | SCD | Oral | NDA; Orphan Drug | TBD |
| gold nanocrystal | Clene | ALS | Oral | NDA; Orphan Drug | TBD |
| ianalumab | Novartis | Autoimmune hemolytic anemia; Sjogren’s syndrome | SC | BLA | TBD |
| imlifidase | Sarepta | Kidney transplant rejection | IV | BLA; Fast Track; Orphan Drug | TBD |
| imsidolimab | Anaptysbio | Generalized pustular psoriasis | IV, SC | BLA; Orphan Drug | TBD |
| inavolisib | Genentech | Breast cancer (HR+/HER2-, 1st-line) | Oral | NDA | TBD |
| IONIS-FB-LRx | Ionis/Roche | IgA nephropathy (Berger’s disease) | SC | NDA | TBD |
| itepekimab | Regeneron | COPD | SC | BLA; Fast Track | TBD |
| JDQ-443 | Novartis | NSCLC | Oral | NDA | TBD |
| ketamine | Hope | Bipolar disorder | IV | NDA; Fast Track | TBD |
| latozinemab | Aldeyra | Frontotemporal dementia | IV | BLA; Breakthrough Therapy; Fast Track; Orphan Drug | TBD |
| lazertinib | Genosco/Janssen | NSCLC | Oral | NDA | TBD |
| lerodalcibep | LIB | Dyslipidemia/hypercholesterolemia; Heterozygous familial hypercholesterolemia (HeFH); Homozygous familial hypercholesterolemia (HoFH) | SC | BLA | TBD |
| leukocyte interleukin | Cel-Sci | SCCHN | SC | BLA; Orphan Drug | TBD |
| ligelizumab | Novartis | Food allergies | SC | BLA | TBD |
| linerixibat | GlaxoSmithKline | Primary biliary cholangitis pruritus | Oral | NDA; Orphan Drug | TBD |
| molgramostim | Savara | Pulmonary alveolar proteinosis | Inhaled | BLA; Breakthrough Therapy; Fast Track; Orphan Drug | TBD |
| navepegritide | Ascendis | Achondroplasia | SC | NDA; Orphan Drug | TBD |
| navitoclax | Abbvie | Myelofibrosis | Oral | NDA; Orphan Drug | TBD |
| nipocalimab | Janssen | Autoimmune hemolytic anemia; Myasthenia gravis | IV | BLA; Fast Track; Orphan Drug | TBD |
| obicetrapib | NewAmsterdam | Dyslipidemia/hypercholesterolemia | Oral | NDA | TBD |
| olezarsen | Akcea | Dyslipidemia/hypercholesterolemia; Familial chylomicronemia syndrome | SC | NDA; Fast Track; Orphan Drug | TBD |
| pabinafusp alfa | JCR | Mucopolysaccharidosis II (MPS II; Hunter Syndrome) | IV | BLA; Orphan Drug | TBD |
| paltusotine | Crinetics | Acromegaly | Oral | NDA; Orphan Drug | TBD |
| pegadricase | Swedish Orphan Biovitrum | Gout | IV | BLA | TBD |
| pegzilarginase | Immedica | Arginase 1 deficiency | IV | BLA; Breakthrough Therapy; Fast Track; Orphan Drug; RPD | TBD |
| pelabresib | Morphosys | Myelofibrosis | Oral | NDA; Fast Track; Orphan Drug | TBD |
| pelacarsen | Novartis | Dyslipidemia/hypercholesterolemia | SC | NDA; Fast Track | TBD |
| piclidenoson | Can-Fite | PSO | Oral | NDA | TBD |
| plozasiran | Arrowhead | Familial chylomicronemia syndrome | SC | NDA; Fast Track; Orphan Drug | TBD |
| ralinepag | United Therapeutics | PAH | Oral | NDA; Orphan Drug | TBD |
| relacorilant | Corcept | Cushing’s syndrome | Oral | NDA; Orphan Drug | TBD |
| remibrutinib | Novartis | MS; Urticaria | Oral | NDA | TBD |
| resiniferatoxin | Grunenthal | Osteoarthritis pain (knee) | Intra-articular | NDA; Breakthrough Therapy | TBD |
| rilzabrutinib | Sanofi | ITP | Oral | NDA; Fast Track; Orphan Drug | TBD |
| rusfertide | Takeda | Polycythemia vera | SC | NDA; Fast Track; Orphan Drug | TBD |
| sebetralstat | Kalvista | HAE | Oral | NDA; Fast Track; Orphan Drug | TBD |
| sepiapterin | PTC | Phenylketonuria (PKU) | Oral | NDA; Orphan Drug | TBD |
| serplulimab | Henlius | SCLC | IV | BLA; Orphan Drug | TBD |
| soticlestat | Takeda | Dravet syndrome; Lennox-Gastaut syndrome | Oral | NDA; Orphan Drug | TBD |
| TAK-279 | Takeda | PSO | Oral | NDA | TBD |
| tamibarotene | Syros | Myelodysplastic syndrome | Oral | NDA; Fast Track; Orphan Drug | TBD |
| telisotuzumab vedotin | Achieve Life Sciences | NSCLC | IV | BLA; Breakthrough Therapy | TBD |
| tiragolumab | Genentech | Esophageal cancer; NSCLC | IV | BLA; Breakthrough Therapy; Orphan Drug | TBD |
| tolebrutinib | Sanofi | MS | Oral | NDA | TBD |
| tiratricol | Rare Thyroid Therapeutics | Resistance to thyroid hormone type beta (RTH-b) | Oral | NDA; Fast Track; Orphan Drug; RPD | TBD |
| vanzacaftor/tezacaftor/deutivacaftor | Vertex | CF | Oral | NDA; Orphan Drug | TBD |
| vatiquinone | PTC | Friedreich’s ataxia | Oral | NDA; Fast Track; Orphan Drug | TBD |
| venglustat | Sanofi | GM2 gangliosidoses (Tay-Sachs disease, Sandhoff disease) | Oral | NDA; Orphan Drug | TBD |
| vimseltinib | Deciphera | Pigmented villonodular synovitis | Oral | NDA; Fast Track | TBD |
| zanidatamab | Jazz | Gastric cancer | IV | BLA; Fast Track; Orphan Drug | TBD |
| zatolmilast | Tetra | Fragile X syndrome | Oral | NDA; Orphan Drug; RPD | TBD |
Phase 3 supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| alpelisib (Piqray®) | Novartis | Breast cancer (HER2+) | Oral | sNDA | TBD |
| atezolizumab (Tecentriq®) | Genentech | Breast cancer (HER2+); Breast cancer (triple-negative) | IV | sBLA | TBD |
| brolucizumab-dbll (Beovu®) | Novartis | Diabetic retinopathy | Intravitreal | sBLA | TBD |
| canakinumab (Ilaris®) | Novartis | NSCLC (adjuvant) | SC | sBLA | TBD |
| cabozantinib (Cabometyx®/Cometriq®) | Exelixis | Prostate cancer | Oral | sNDA | TBD |
| cemiplimab-rwlc (Libtayo®) | Regeneron | Melanoma | IV | sBLA; Fast Track | TBD |
| dupilumab (Dupixent®) | Sanofi | Bullous pemphigoid | SC | sBLA; Orphan Drug | TBD |
| durvalumab (Imfinzi®) | AstraZeneca | Bladder cancer (1st-line) | IV | sBLA; Breakthrough Therapy | TBD |
| eplontersen (Wainua™) | Ionis | Transthyretin amyloid cardiomyopathy | SC | sNDA; Fast Track; Orphan Drug | TBD |
| ganaxolone (Ztalmy®) | Marinus | Status epilepticus (refractory); Tuberous sclerosis complex | IV, Oral | sNDA; Orphan Drug | TBD |
| iptacopan (Fabhalta®) | Novartis | C3 glomerulopathy; Hemolytic uremic syndrome; IgA nephropathy (Berger’s disease) | Oral | sNDA; Breakthrough Therapy; Orphan Drug; RPD | TBD |
| mepolizumab (Nucala®) | GlaxoSmithKline | COPD | IV, SC | sBLA | TBD |
| mirikizumab-mrkz (Omvoh™) | Eli Lilly | CD | IV, SC | sBLA | TBD |
| mitapivat (Pyrukynd®) | Agios | SCD; Thalassemia | Oral | sNDA; Orphan Drug | TBD |
| mosunetuzumab-axgb (Lunsumio®) | Genentech | DLBCL | SC | sBLA | TBD |
| obinutuzumab (Gazyva®) | Genentech | Lupus nephritis; Membranous nephropathy; SLE | IV | sBLA; Breakthrough Therapy | TBD |
| pozelimab (Veopoz™) | Regeneron | PNH | IV, SC | sBLA; Orphan Drug | TBD |
| ranibizumab port delivery system (Susvimo®) | Genentech | DME | Intravitreal implant | sBLA | TBD |
| satralizumab-mwge (Enspryng®) | Genentech | Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) | SC | sBLA; Orphan Drug | TBD |
| secukinumab (Cosentyx®) | Novartis | Giant cell arteritis; Lupus nephritis | SC | sBLA | TBD |
| sotorasib (Lumakras®) | Amgen | CRC | Oral | sNDA; Orphan Drug | TBD |
| venetoclax (Venclexta®) | Abbvie/Genenetech | Myelodysplastic syndrome; Multiple myeloma | Oral | sNDA; Breakthrough Therapy; Orphan Drug | TBD |
| vutrisiran (Amvuttra®) | Alnylam | Transthyretin amyloid cardiomyopathy | SC | sNDA; Orphan Drug | TBD |
Traditional
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| levodopa/carbidopa patch pump | Mitsubishi Tanabe | Parkinson’s disease | SC infusion | 505(b)(2) NDA | Apr-Jun 2024 |
| pivmecillinam | Utility | UTI (uncomplicated) | Oral | NDA; Priority Review; QIDP | 4/24/2024 |
| RSV pre-fusion F protein vaccine (mRNA-1345) | Moderna | RSV-related lower respiratory tract illness prevention (ages > 60 years) | IM | BLA; Breakthrough Therapy; Fast Track; Priority Review | 05/10/2024 |
| sofpironium | Botanix | Axillary hyperhidrosis (severe) | Topical | NDA | June 2024 |
| pneumococcal polyvalent conjugate vaccine | Merck | Pneumococcal immunization (adults) | IM | BLA; Breakthrough Therapy; Priority Review | 06/17/2024 |
| ensifentrine | Verona | COPD | Inhaled | NDA | 06/26/2024 |
| insulin icodec | Novo Nordisk | T1DM; T2DM | SC | BLA | Jul-Sep 2024 |
| naloxone (powder-based technology) | Orexo | Opioid overdose | Intranasal | 505(b)(2) NDA | 07/15/2024 |
| galantamine | Alpha Cognition | Alzheimer’s disease (mild-moderate) | Oral | 505(b)(2) NDA | 07/27/2024 |
| carbidopa/levodopa ER | Amneal | Parkinson’s disease | Oral | 505(b)(2) NDA | 08/08/2024 |
| tradipitant | Vanda | Gastroparesis | Oral | NDA | 09/18/2024 |
| xanomeline/trospium | Karuna | Schizophrenia | Oral | NDA | 09/26/2024 |
| epinephrine | ARS | Anaphylaxis | Intranasal | 505(b)(2) NDA; Fast Track | 10/02/2024 |
| minocycline | Journey | Rosacea | Oral | 505(b)(2) NDA | 11/04/2024 |
| meningococcal vaccine (GSK3536819A) | GlaxoSmithKline | Meningococcal immunization | IM | BLA | 02/14/2025 |
| etripamil | Milestone | Paroxysmal supraventricular tachycardia | Intranasal | NDA | 03/28/2025 |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| diazepam buccal film (Libervant™) | Aquestive | Seizure disorders (ages 2 to 5 years) | Oral transmucosal | sNDA; Fast Track; Orphan Drug | 04/26/2024 |
| hepatitis B vaccine (recombinant), adjuvanted (Heplisav-B®) | Dynavax | Hepatitis B immunization (adults on hemodialysis) | IM | sBLA | 05/13/2024 |
| RSV vaccine, adjuvanted (Arexvy™) | GlaxoSmithKline | RSV prevention (ages 50 to 59 years) | IM | sBLA; Fast Track; Priority Review | 06/07/2024 |
| roflumilast (Zoryve®) | Arcutis | Atopic dermatitis (adults and pediatrics ages ≥ 6 years) | Topical | sNDA | 07/07/2024 |
| vonoprazan (Takecab®) | Phathom | GERD (non-erosive) | Oral | sNDA | 07/19/2024 |
| anacaulase-bcdb (Nexobrid®) | Vericel | Burn injury (pediatrics) | Topical | sNDA; Orphan Drug | 11/08/2024 |
| semaglutide (Wegovy®) | Novo Nordisk | Chronic HFpEF | SC | sNDA | 11/29/2024 |
| tapinarof (Vtama®) | Dermavant | Atopic dermatitis (ages ≥ 2 years) | Topical | sNDA | December 2024 |
| brexpiprazole (Rexulti®) | Otsuka | PTSD (in combination with sertraline) | Oral | sNDA | 02/07/2025 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| aceclidine | LENZ | Presbyopia | Ophthalmic | NDA | TBD |
| AR-15512 | Aerie | DED | Ophthalmic | NDA | TBD |
| aroxybutynin/atomoxetine | Apnimed | Sleep apnea | Oral | NDA; Fast Track | TBD |
| baclofen/naltrexone/sorbitol | Pharnext | Charcot-Marie-Tooth disease | Oral | NDA | TBD |
| bemnifosbuvir | Atea | COVID-19 | Oral | NDA; Fast Track | TBD |
| bentracimab | SERB | Ticagrelor (Brilinta®) reversal | IV | BLA; Breakthrough Therapy | TBD |
| blarcamesine | Anavex | Alzheimer’s disease | Oral | NDA | TBD |
| brilaroxazine | Reviva | Schizophrenia | Oral | NDA | TBD |
| cagrilintide/semaglutide | Novo Nordisk | Obesity/overweight; T2DM | SC | NDA | TBD |
| carbachol/brimonidine | Visus | Presbyopia | Ophthalmic | 505(b)(2) NDA | TBD |
| chikungunya vaccine | Bavarian Nordic | Chikungunya virus prevention | IM | BLA; Breakthrough Therapy; Fast Track | TBD |
| cytisinicline | Achieve Life Sciences | Smoking cessation | Oral | NDA | TBD |
| dengue tetravalent vaccine, live, attenuated | Takeda | Dengue fever (ages 4-60 years) | SC | BLA; Fast Track | TBD |
| elinzanetant | Bayer | Menopausal vasomotor symptoms | Oral | NDA | TBD |
| ensitrelvir fumaric acid | Shionogi | COVID-19 | Oral | BLA; Fast Track | TBD |
| epinephrine (sublingual) | Aquestive | Anaphylaxis | SL | 505(b)(2) NDA; Fast Track | TBD |
| esreboxetine | Axsome | Fibromyalgia | Oral | NDA | TBD |
| estetrol | Mithra | Menopausal vasomotor symptoms | Oral | NDA | TBD |
| gepotidacin | GlaxoSmithKline | Urogenital gonorrhea | Oral | NDA; QIDP | TBD |
| navacaprant | Neumora | MDD | Oral | NDA | TBD |
| orforglipron | Eli Lilly | Obesity/overweight; T2DM | Oral | NDA | TBD |
| paromomycin | Appili | Leishmaniasis | Topical | NDA; Orphan Drug | TBD |
| PL-9643 | Palatin | DED | Ophthalmic | NDA | TBD |
| purified vero rabies vaccine (SP0087) | Sanofi | Rabies | IM | BLA | TBD |
| QRX003 | Quoin | Congenital ichthyosis; Netherton syndrome | Topical | NDA | TBD |
| quadrivalent influenza mRNA vaccine (mRNA-1010) | Moderna | Seasonal influenza immunization | IM | BLA | TBD |
| retatrutide | Eli Lilly | Obesity/overweight; T2DM | SC | NDA | TBD |
| sulopenem etzadroxil/probenecid | Iterum | UTI (uncomplicated) | Oral | NDA; Fast Track; QIDP | TBD |
| survodutide | Boehringer Ingelheim | Obesity/overweight; T2DM | SC | NDA | TBD |
| suzetrigine | Vertex | Postsurgical pain | Oral | NDA; Breakthrough Therapy; Fast Track | TBD |
| tramiprosate | Alzheon | Alzheimer’s disease | Oral | NDA; Fast Track | TBD |
| ulixacaltamide | Praxis | Essential tremor | Oral | NDA | TBD |
| VHX-896 | Vanda | Bipolar disorder; Schizophrenia | Oral | NDA | TBD |
| XEN1101 | Xenon | Partial/focal seizures | Oral | NDA | TBD |
| zoliflodacin | Innoviva | gonorrhea (uncomplicated) | Oral | NDA; Fast Track; QIDP | TBD |
Phase 3 supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| ibrexafungerp (Brexafemme®) | GlaxoSmithKline | Fungal infections (systemic) | Oral | sNDA; Fast Track; Orphan Drug; QIDP | TBD |
| lumateperone (Caplyta®) | Intra-Cellular Therapies | MDD | Oral | sNDA | TBD |
| phentolamine 0.75% (Ryzumvi™) | Ocuphire | Presbyopia | Ophthalmic | sNDA | TBD |
| roflumilast (Zoryve®) | Arcutis | PSO | Topical | sNDA | TBD |
| semaglutide (Ozempic®) | Novo Nordisk | Diabetic nephropathy | SC | sNDA | TBD |
| semaglutide (Rybelsus®) | Novo Nordisk | Obesity/overweight | Oral | sNDA | TBD |
Submitted new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| levodopa/carbidopa patch pump | Mitsubishi Tanabe | Parkinson’s disease | SC infusion | 505(b)(2) NDA | Apr-Jun 2024 |
| pivmecillinam | Utility | UTI (uncomplicated) | Oral | NDA; Priority Review; QIDP | 4/24/2024 |
| RSV pre-fusion F protein vaccine (mRNA-1345) | Moderna | RSV-related lower respiratory tract illness prevention (ages > 60 years) | IM | BLA; Breakthrough Therapy; Fast Track; Priority Review | 05/10/2024 |
| sofpironium | Botanix | Axillary hyperhidrosis (severe) | Topical | NDA | June 2024 |
| pneumococcal polyvalent conjugate vaccine | Merck | Pneumococcal immunization (adults) | IM | BLA; Breakthrough Therapy; Priority Review | 06/17/2024 |
| ensifentrine | Verona | COPD | Inhaled | NDA | 06/26/2024 |
| insulin icodec | Novo Nordisk | T1DM; T2DM | SC | BLA | Jul-Sep 2024 |
| naloxone (powder-based technology) | Orexo | Opioid overdose | Intranasal | 505(b)(2) NDA | 07/15/2024 |
| galantamine | Alpha Cognition | Alzheimer’s disease (mild-moderate) | Oral | 505(b)(2) NDA | 07/27/2024 |
| carbidopa/levodopa ER | Amneal | Parkinson’s disease | Oral | 505(b)(2) NDA | 08/08/2024 |
| tradipitant | Vanda | Gastroparesis | Oral | NDA | 09/18/2024 |
| xanomeline/trospium | Karuna | Schizophrenia | Oral | NDA | 09/26/2024 |
| epinephrine | ARS | Anaphylaxis | Intranasal | 505(b)(2) NDA; Fast Track | 10/02/2024 |
| minocycline | Journey | Rosacea | Oral | 505(b)(2) NDA | 11/04/2024 |
| meningococcal vaccine (GSK3536819A) | GlaxoSmithKline | Meningococcal immunization | IM | BLA | 02/14/2025 |
| etripamil | Milestone | Paroxysmal supraventricular tachycardia | Intranasal | NDA | 03/28/2025 |
Submitted supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| diazepam buccal film (Libervant™) | Aquestive | Seizure disorders (ages 2 to 5 years) | Oral transmucosal | sNDA; Fast Track; Orphan Drug | 04/26/2024 |
| hepatitis B vaccine (recombinant), adjuvanted (Heplisav-B®) | Dynavax | Hepatitis B immunization (adults on hemodialysis) | IM | sBLA | 05/13/2024 |
| RSV vaccine, adjuvanted (Arexvy™) | GlaxoSmithKline | RSV prevention (ages 50 to 59 years) | IM | sBLA; Fast Track; Priority Review | 06/07/2024 |
| roflumilast (Zoryve®) | Arcutis | Atopic dermatitis (adults and pediatrics ages ≥ 6 years) | Topical | sNDA | 07/07/2024 |
| vonoprazan (Takecab®) | Phathom | GERD (non-erosive) | Oral | sNDA | 07/19/2024 |
| anacaulase-bcdb (Nexobrid®) | Vericel | Burn injury (pediatrics) | Topical | sNDA; Orphan Drug | 11/08/2024 |
| semaglutide (Wegovy®) | Novo Nordisk | Chronic HFpEF | SC | sNDA | 11/29/2024 |
| tapinarof (Vtama®) | Dermavant | Atopic dermatitis (ages ≥ 2 years) | Topical | sNDA | December 2024 |
| brexpiprazole (Rexulti®) | Otsuka | PTSD (in combination with sertraline) | Oral | sNDA | 02/07/2025 |
Phase 3 new drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| aceclidine | LENZ | Presbyopia | Ophthalmic | NDA | TBD |
| AR-15512 | Aerie | DED | Ophthalmic | NDA | TBD |
| aroxybutynin/atomoxetine | Apnimed | Sleep apnea | Oral | NDA; Fast Track | TBD |
| baclofen/naltrexone/sorbitol | Pharnext | Charcot-Marie-Tooth disease | Oral | NDA | TBD |
| bemnifosbuvir | Atea | COVID-19 | Oral | NDA; Fast Track | TBD |
| bentracimab | SERB | Ticagrelor (Brilinta®) reversal | IV | BLA; Breakthrough Therapy | TBD |
| blarcamesine | Anavex | Alzheimer’s disease | Oral | NDA | TBD |
| brilaroxazine | Reviva | Schizophrenia | Oral | NDA | TBD |
| cagrilintide/semaglutide | Novo Nordisk | Obesity/overweight; T2DM | SC | NDA | TBD |
| carbachol/brimonidine | Visus | Presbyopia | Ophthalmic | 505(b)(2) NDA | TBD |
| chikungunya vaccine | Bavarian Nordic | Chikungunya virus prevention | IM | BLA; Breakthrough Therapy; Fast Track | TBD |
| cytisinicline | Achieve Life Sciences | Smoking cessation | Oral | NDA | TBD |
| dengue tetravalent vaccine, live, attenuated | Takeda | Dengue fever (ages 4-60 years) | SC | BLA; Fast Track | TBD |
| elinzanetant | Bayer | Menopausal vasomotor symptoms | Oral | NDA | TBD |
| ensitrelvir fumaric acid | Shionogi | COVID-19 | Oral | BLA; Fast Track | TBD |
| epinephrine (sublingual) | Aquestive | Anaphylaxis | SL | 505(b)(2) NDA; Fast Track | TBD |
| esreboxetine | Axsome | Fibromyalgia | Oral | NDA | TBD |
| estetrol | Mithra | Menopausal vasomotor symptoms | Oral | NDA | TBD |
| gepotidacin | GlaxoSmithKline | Urogenital gonorrhea | Oral | NDA; QIDP | TBD |
| navacaprant | Neumora | MDD | Oral | NDA | TBD |
| orforglipron | Eli Lilly | Obesity/overweight; T2DM | Oral | NDA | TBD |
| paromomycin | Appili | Leishmaniasis | Topical | NDA; Orphan Drug | TBD |
| PL-9643 | Palatin | DED | Ophthalmic | NDA | TBD |
| purified vero rabies vaccine (SP0087) | Sanofi | Rabies | IM | BLA | TBD |
| QRX003 | Quoin | Congenital ichthyosis; Netherton syndrome | Topical | NDA | TBD |
| quadrivalent influenza mRNA vaccine (mRNA-1010) | Moderna | Seasonal influenza immunization | IM | BLA | TBD |
| retatrutide | Eli Lilly | Obesity/overweight; T2DM | SC | NDA | TBD |
| sulopenem etzadroxil/probenecid | Iterum | UTI (uncomplicated) | Oral | NDA; Fast Track; QIDP | TBD |
| survodutide | Boehringer Ingelheim | Obesity/overweight; T2DM | SC | NDA | TBD |
| suzetrigine | Vertex | Postsurgical pain | Oral | NDA; Breakthrough Therapy; Fast Track | TBD |
| tramiprosate | Alzheon | Alzheimer’s disease | Oral | NDA; Fast Track | TBD |
| ulixacaltamide | Praxis | Essential tremor | Oral | NDA | TBD |
| VHX-896 | Vanda | Bipolar disorder; Schizophrenia | Oral | NDA | TBD |
| XEN1101 | Xenon | Partial/focal seizures | Oral | NDA | TBD |
| zoliflodacin | Innoviva | gonorrhea (uncomplicated) | Oral | NDA; Fast Track; QIDP | TBD |
Phase 3 supplemental drugs
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status | FDA Decision |
| ibrexafungerp (Brexafemme®) | GlaxoSmithKline | Fungal infections (systemic) | Oral | sNDA; Fast Track; Orphan Drug; QIDP | TBD |
| lumateperone (Caplyta®) | Intra-Cellular Therapies | MDD | Oral | sNDA | TBD |
| phentolamine 0.75% (Ryzumvi™) | Ocuphire | Presbyopia | Ophthalmic | sNDA | TBD |
| roflumilast (Zoryve®) | Arcutis | PSO | Topical | sNDA | TBD |
| semaglutide (Ozempic®) | Novo Nordisk | Diabetic nephropathy | SC | sNDA | TBD |
| semaglutide (Rybelsus®) | Novo Nordisk | Obesity/overweight | Oral | sNDA | TBD |
Complete response letter
| Name | Manufacturer | Clinical Use | Dosage Form | Development Status |
| apomorphine | Supernus | Parkinson’s disease (continuous treatment of motor fluctuations) | SC infusion | CRL |
| cefepime/taniborbactam | Venatorx/Melinta | UTI (complicated) | IV | CRL |
| dihydroergotamine nasal powder | Shin Nippon | Migraine (acute treatment) | Intranasal | CRL |
| odronextamab | Pfizer | DLBCL (R/R); Follicular lymphoma (R/R) | IV | CRL |
| roluperidone | Minerva | Schizophrenia (negative symptoms) | Oral | CRL |
| scopolamine | Repurposed Therapeutics | Motion sickness | Intranasal | CRL |
| tasimelteon (Hetlioz®) | Vanda | Jet lag disorder | Oral | CRL |
| tesamorelin (Egrifta®) high concentration | Theratechnologies | HIV lipodystrophy | SC | CRL |
Glossary of terms
Glossary of terms
5-FU 5-Fluorouracil
6MWD 6 Minute Walking Distance
6MWT 6 Minute Walking Test
ABSSSI Acute Bacterial Skin and Skin Structure Infection
ACC American College of Cardiology
ACEI Angiotensin-Converting Enzyme Inhibitor
ACR20 American College of Rheumatology 20% Improvement
ACR50 American College of Rheumatology 50% Improvement
ACR70 American College of Rheumatology 70% Improvement
ADC Antibody-Drug Conjugate
ADHD Attention Deficit Hyperactivity Disorder
ADL Activities of Daily Living
ALK Anaplastic Lymphoma Kinase
ALK+ Anaplastic Lymphoma Kinase-positive
ALL Acute Lymphoblastic Leukemia
ALS Amyotrophic Lateral Sclerosis
ALSFRS-R Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised
ALT Alanine Transaminase
AMD Age-Related Macular Degeneration
AML Acute Myeloid Leukemia
ANCA Antineutrophil Cytoplasmic Antibodies
ARB Angiotensin II Receptor Blocker
ARNI Angiotensin Receptor II Blocker – Neprilysin Inhibitor
AS Ankylosing Spondylitis
ASCVD Atherosclerotic Cardiovascular Disease
AST Aspartate Aminotransferase
BCG Bacillus Calmette-Guérin
BCL-1
BCVA Best Corrected Visual Acuity
BLA Biologics License Application
BMI Body Mass Index
BMT Bone Marrow Transplant
BP Blood Pressure
BPH Benign Prostatic Hyperplasia
BRAF V-Raf Murine Sarcoma Viral Oncogene Homolog B1
BTK Bruton’s Tyrosine Kinase
BSA Body Surface Area
BsUFA Biosimilar User Fee Act
CABP Community Acquired Bacterial Pneumonia
CAP Community Acquired Pneumonia
CAR T Chimeric Antigen Receptor T-Cell
CD Crohn’s Disease
CD3 Cluster of Differentiate 3
CD19 Cluster of Differentiate 19
CD20 Cluster of Differentiate 20
CD38 Cluster of Differentiate 38
CD79b Cluster of Differentiate 79b
CDC Centers for Disease Control and Prevention
CF Cystic Fibrosis
CHF Congestive Heart Failure
CI Confidence Interval
CKD Chronic Kidney Disease
CLL Chronic Lymphocytic Leukemia
CML Chronic Myeloid Leukemia
CMS Centers for Medicare & Medicaid Services
CNS Central Nervous System
COPD Chronic Obstructive Pulmonary Disease
COVID-19 Coronavirus Disease 2019
CRC Colorectal Cancer
CRL Complete Response Letter
CRR Complete Response Rate
CRS Cytokine Release Syndrome
CSF Colony Stimulating Factor
CTLA-4 Cytotoxic T-Lymphocyte-Associated Protein 4
CV Cardiovascular
CVD Cardiovascular Disease
CYP3A4 Cytochrome P-450 3A4
CYP450 Cytochrome P-450
DAS28-CRP Disease Activity Score-28 with C Reactive Protein
DBP Diastolic Blood Pressure
DCR Disease Control Rate
DEA Drug Enforcement Administration
DED Dry Eye Disease
DLBCL Diffuse Large B Cell Lymphoma
DMARD Disease Modifying Antirheumatic Drug
DMD Duchenne Muscular Dystrophy
DME Diabetic Macular Edema
dMMR DNA mismatch repair
DMT Disease Modifying Therapy
DNA Deoxyribonucleic Acid
DOR Duration of Response
DPP-4 Dipeptidyl Peptidase 4
DR Delayed-Release
DSM-5 Diagnostic and Statistical Manual of Mental Disorders, 5th edition
EASI-75 Eczema Area and Severity Index ≥ 75% Reduction
ECOG Eastern Cooperative Oncology Group
EDSS Expanded Disability Status Scale
eGFR estimated Glomerular Filtration Rate
EGFR Epidermal Growth Factor Receptor
ER Extended-Release
ERA Endothelin Receptor Agonist
ESA Erythropoietin Stimulating Agent
ESRD End-Stage Renal Disease
EUA Emergency Use Authorization
FDA Food and Drug Administration
FEV1 Force Expiratory Volume in 1 Second
FH Familial Hypercholesterolemia
FLT3 FMS-Like Tyrosine Kinase-3
FMS Feline McDonough Sarcoma
FVC Forced Vital Capacity
GABA-A Gamma-Aminobutyric Acid Receptor Type A
G-CSF Granulocyte Colony Stimulating Factor
GERD Gastroesophageal Reflux Disease
GGT Gamma-Glutamyl Transferase
GI Gastrointestinal
GIST Gastrointestinal Stromal Tumor
GLP-1RA Glucagon-Like Peptide-1 Receptor Agonist
GM-CSF Granulocyte-Macrophage Colony Stimulating Factor
GVHD Graft Versus Host Disease
H Half
HAE Hereditary Angioedema
HAM-A Hamilton Anxiety Rating Scale
HAM-D Hamilton Depression Rating Scale
HAMD-17 Hamilton Depression Rating Scale
HAP Healthcare-Associated Pneumonia
Hb Hemoglobin
HbA1c Hemoglobin A1c
HBV Hepatitis B Virus
HCC Hepatocellular Carcinoma
HCP Healthcare Professional
HCV Hepatitis C Virus
HDRS-17 Hamilton Depression Rating Scale
HER Human Epidermal Growth Factor Receptor
HER2 Human Epidermal Growth Factor Receptor 2
HER2- Human Epidermal Growth Factor Receptor 2-negative
HER2+ Human Epidermal Growth Factor Receptor 2-positive
HF Heart Failure
HFA Hydrofluoroalkane
HFpEF Heart Failure with preserved Ejection Fraction
HFrEF Heart Failure with reduced Ejection Fraction
HIT Heparin Induced Thrombocytopenia
HIV Human Immunodeficiency Virus
HIV-1 Human Immunodeficiency Virus-1
HR Hazard Ratio
HR+ Hormone Receptor-positive
HS Hidradenitis Suppurativa
HSCT Hematopoietic Stem Cell Transplant
HSV Herpes Simplex Virus
HTN Hypertension
IBS Irritable Bowel Syndrome
IBS-C Irritable Bowel Syndrome, Constipation Predominant
ICER Institute for Clinical and Economic Review
ICS Inhaled Corticosteroid
IDH Isocitrate Dehydrogenase
IGA Investigator’s Global Assessment
IgG Immunoglobulin G
IgG1 Immunoglobulin G1
IHC Immunohistochemistry
IL-4 Interleukin-4
IL-5 Interleukin-5
IL-8 Interleukin-8
IL-12 Interleukin-12
IL-13 Interleukin-13
IL-17 Interleukin-17
IL-23 Interleukin-23
IL-31 Interleukin-31
IM Intramuscular
IR Immediate-Release
IRB Institutional Review Board
ITP Immune Thrombocytopenic Purpura
ITT Intent-To-Treat
IV Intravenous
JAK Janus Kinase Inhibitor
JIA Juvenile Idiopathic Arthritis
KIT c-KIT Proto-Oncogene
KMT2A Lysine (K)-Specific Methyltransferase 2A
LABA Long-Acting Beta Agonist
LAMA Long-Acting Muscarinic Antagonist
LDL-C Low-Density Lipoprotein Cholesterol
LPAD Limited Population Pathway for Antibacterial and Antifungal Drugs
LS Least Square
LVEF Left Ventricular Ejection Fraction
mAb Monoclonal Antibody
MACE Major Adverse Cardiovascular Events
MADRS Montgomery – Åsberg Depression Rating Scale
MDD Major Depressive Disorder
MDI Metered Dose Inhaler
MDR Multi-Drug Resistant
MECP2 Methyl-CpG Binding Protein 2
MEK Mitogen-Activated Extracellular Signal-Regulated Kinase
MI Myocardial Infarction
mITT modified Intent-To-Treat
MRI Magnetic Resonance Imaging
MRSA Methicillin-Resistant Staphylococcus Aureus
MS Multiple Sclerosis
MSI-H Microsatellite Instability-High
N/A Not Applicable
NAFLD Nonalcoholic Fatty Liver Disease
NASH Non-Alcoholic Steatohepatitis
NCCN National Comprehensive Cancer Network
NCT National Clinical Trials
NDA New Drug Application
NHL Non-Hodgkin Lymphoma
NIH National Institutes of Health
NRAS Neuroblastoma RAS Proto-Oncogene
NSAID Non-Steroidal Anti-Inflammatory Drug
NSCLC Non-Small Cell Lung Cancer
NTRK Neurotrophic Tyrosine Receptor Kinase
NYHA New York Heart Association
ODT Orally Disintegrating Tablet
OR Odds Ratio
ORR Objective Response Rate
OS Overall Survival
OTC Over-the-Counter
PAD Peripheral Arterial Disease
PAH Pulmonary Arterial Hypertension
PARP Poly (ADP-Ribose) Polymerase
PAS Prior Approval Supplement
PASI Psoriasis Area and Severity Index
PASI 50 Psoriasis Area and Severity Index 50% Reduction
PASI 75 Psoriasis Area and Severity Index 75% Reduction
PASI 90 Psoriasis Area and Severity Index 90% Reduction
PASI 100 Psoriasis Area and Severity Index 100% Reduction
PCI Percutaneous Coronary Intervention
PCSK9 Proprotein Convertase Subtilisin Kexin 9
PD-1 Programmed Death Protein 1
PD-L1 Programmed Death-Ligand 1
PDE3 Phosphodiesterase 3
PDE4 Phosphodiesterase 4
PDE5 Phosphodiesterase 5
PDUFA Prescription Drug User Fee Application
PFS Progression-Free Survival
PGA Physician Global Assessment
PHI Primary Humoral Immunodeficiency
PI3K Phosphatidylinositol-3-kinase
PNH Paroxysmal Nocturnal Hemoglobinuria
PsA Psoriatic Arthritis
PSO Plaque Psoriasis
PTCA Percutaneous Transluminal Coronary Angioplasty
PTSD Post-Traumatic Stress Disorder
Q Quarter
QIDP Qualified Infectious Diseases Product
QOL Quality of Life
R/R Relapsed or Refractory
R-CHOP Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisone
RA Rheumatoid Arthritis
RAS Ras Protein Superfamily
RBC Red Blood Cell
RCC Renal Cell Carcinoma
REMS Risk Evaluation and Mitigation Strategy
RMAT Regenerative Medicine Advanced Therapy
RNA Ribonucleic Acid
ROS1 ROS Proto-Oncogene 1
RPD Rare Pediatric Disease
RRR Relative Risk Reduction
RSV Respiratory Syncytial Virus
RTOR Real-Time Oncology Review
RVO Retinal Vein Occlusion
SARS-CoV-2 Severe Acute Respiratory Syndrome-Associated Coronavirus-2
sBLA supplemental Biologics License Application
SBP Systolic Blood Pressure
SC Subcutaneous
SCCHN Squamous Cell Cancer of the Head and Neck
SCD Sickle Cell Disease
SCLC Small Cell Lung Cancer
SCT Stem Cell Transplant
SGLT2 Sodium-Glucose Co-Transporter 2
SL Sublingual
SLE Systemic Lupus Erythematosus
SLL Small Lymphocytic Lymphoma
sNDA supplemental New Drug Application
SNRI Serotonin and Norepinephrine Reuptake Inhibitor
SOC Standard of Care
SOD-1 Superoxide Dismutase – Type 1
sPGA static Physician Global Assessment
SR Sustained-Release
SSRI Selective Serotonin Reuptake Inhibitor
SSSI Skin and Skin Structure Infection
T1DM Type 1 Diabetes Mellitus
T2DM Type 2 Diabetes Mellitus
TBD To Be Determined
TEAE Treatment-Emergent Adverse Event
TKI Tyrosine Kinase Inhibitor
TNBC Triple Negative Breast Cancer
TNF Tumor Necrosis Factor
TNFα Tumor Necrosis Factor-alpha
UA Unstable Angina
UC Ulcerative Colitis
ULN Upper Limit of Normal
U.S. United States
UTI Urinary Tract Infection
VAS Visual Analog Scale
VEGF Vascular Endothelial Growth Factor
VTE Venous Thromboembolism
WBC White Blood Cell
WHO World Health Organization
XR Extended-Release
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts