Stories
Prime pharmacists address AMCP virtual conference as keynote speakers
Catherine Starner, PharmD and Nicole Kjesbo, PharmD discuss the specialty drug pipeline and forecast
April 23, 2021To determine the pipeline and forecast, Starner and Kjesbo consider cost, population/prevalence and management strategies – such as utilization management, prior authorizations and quantity limits. For inclusion on the Watch List, Prime generally focuses on drugs that are submitted to the U.S. Food and Drug Administration (FDA) and have a high anticipated spend based on per member per month (PMPM). Prime forecasts drugs on both medical and pharmacy benefits to help manage total drug spend for its health plan clients.
Starner and Kjesbo focused on pipeline and forecasting for:
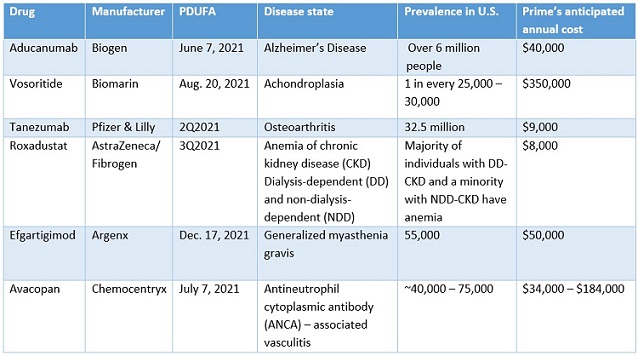
While gene therapy approvals have been few and far between, there are more on the horizon. Possible gene therapy reviews include:
- Eladocagene exuparvovec, PTC Therapeutics – aromatic l-amino acid decarboxylase (AADC) deficiency
- SRP-9001, Sarepta Therapeutics – Duchenne muscular dystrophy (DMD)
- AMT-061 (etranacogene dezaparvovec), uniQure – hemophilia B
Prime publishes monthly decisions expected from the FDA and monthly pipelines.
Related news
Stories
July 22, 2024
Introducing the latest issue of our medical and pharmacy benefit management report
The summer 2024 issue of the Magellan Rx Report provides insights on the industry’s most groundbreaking managed care solutions in some of the most complex areas of health care
Stories
July 18, 2024
Specialty drug trend forecasted to drop for employer groups, clinical experts say
Introduction of Humira® and Stelara® biosimilars contribute to continued decline in the autoimmune – anti-inflammatory trend
Stories
July 16, 2024
Prime/Magellan Rx earns top score on the Disability Equality Index
For the second consecutive year, Prime Therapeutics/Magellan Rx Management received a score of 100 on the Disability Equality Index®, which benchmarks disability workplace inclusion