Perspectives
Preparing clients for gene therapy sticker shock
PreserveRx can give Blue Plans the confidence to plan for the inevitable future of gene therapy.
March 22, 2022When Prime helps health plan clients get ready for cost increases from a high cost drug, one of the tools we use is looking at the new drug cost spread out over the entire member population. Prime’s Blue Plan client commercial population is 16 million members, so that’s a common metric we use.
For Prime’s Drug Watch List, we pay particular attention to any drug that is going to raise the per member per month (PMPM) cost (across 16 million members) more than $0.08. At $0.08 PMPM, that increase will have a significant impact on the year’s drug trend.
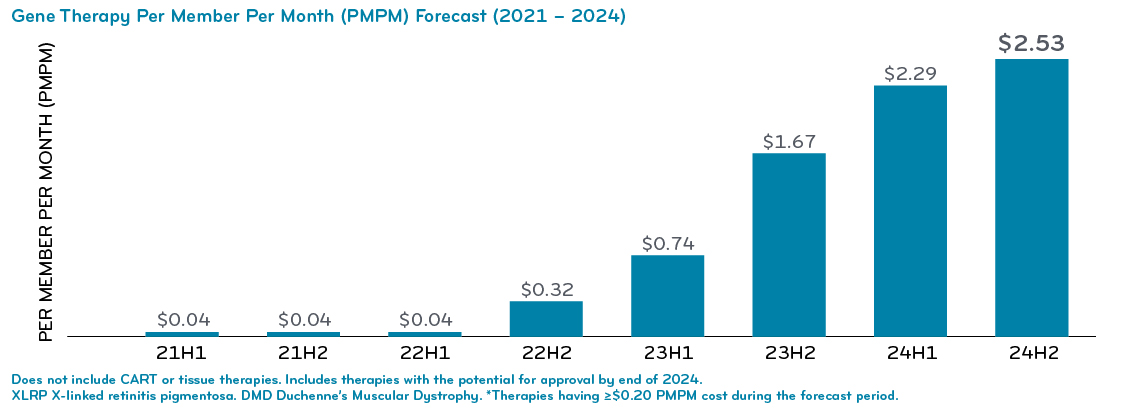
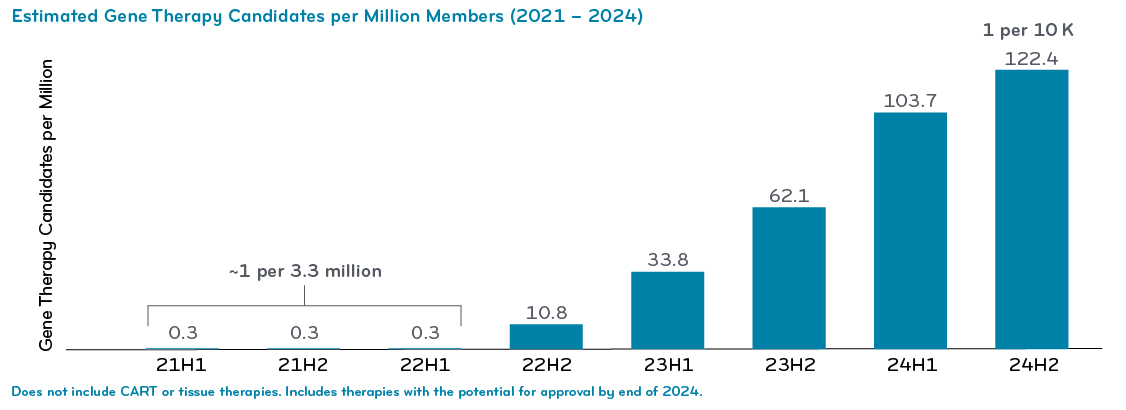
We’ve been watching the gene therapy pipeline very closely. So far, there are just two gene therapies on the market now. Prime calculates that to be $0.04 per member per month (PMPM). But in two short years, a potential for 18 more gene therapies could bring that up to $2.53 PMPM.
These therapies have price tags up to $3 and $4 million each. For a 10,000 member self-insured group, having a member with one of these claims in a given year is not $2.53 PMPM – it’s $16.67 PMPM. That goes beyond significant, to potentially devastating.
- This article will describe the work Prime does to forecast the impact of these costly gene therapy drugs.
- We‘ll show our gene therapy forecast for the next three years. We’ll show that breakdown by PMPM and also by numbers of patients per million members.
- Then we’ll explain how PreserveRx, a reinsurance product can help Blue Plans keep these gene therapies accessible to members while working to contain the cost impact.
Definition: Reinsurance is insurance that an insurance company purchases from another insurance company to insulate itself from the risk of a major claims event. With reinsurance, the company passes on some part of its own insurance liabilities to the other insurance company.
Definition: Attachment point is extra insurance coverage, bought in addition to primary reinsurance coverage, that applies if the claim goes above a certain dollar amount.
Prime practices “no surprises” pipeline monitoring
Gene therapy treatments bring highly concentrated costs across just a few members. These costs are not evenly distributed. That’s where the risk is. How does a plan prepare? Prime can do a lot of preparation work before a gene therapy is even approved.

Prime has a dedicated team of specialized pharmacists and data scientists that comes together to help monitor the drug pipeline and analyze our 16 million members’ medical and pharmacy claims to answer the questions above and prevent pipeline surprises.
Definition: PDUFA stands for the Prescription Drug User Fee Act (PDUFA), created by Congress in 1992. It authorizes the FDA to collect user fees from drug companies as part of expediting the drug approval process. Over time, the acronym and shorthand, PDUFA date, has come to refer to the scheduled date when the FDA will announce its decision on whether a drug is approved or not.
Definition: BLA, a biologic license application, is submitted with a biologic drug product to the Food and Drug Administration (FDA) requesting approval for manufacturing and distribution. A BLA includes applicant information, product/manufacturing information, pre-clinical studies, clinical studies and labeling.
We monitor active clinical trials. These help inform us where the drugs are in their timeline. The trials show us the inclusion/exclusion criteria of the patients being treated with that therapy. We can use those markers to do our medical and pharmacy claims data analytics: we crosswalk back to members in the plan population who could be potentially treated by these therapies. That helps us identify potential patient populations.
We look at investment reports. Those give us additional clues for the approval timelines. Sometimes investment analysts have price tags that they are estimating for these therapies as well.
Manufacturers provide information. Press releases often include PDUFA dates, clinical holds, and information about BLA filings for timing. We lean on our manufacturer relationships to provide clinical or operational context. They will have assigned someone to work with Prime and answer questions. Prime may ask for a clinical presentation from the manufacturer.
We study medical literature. We supplement this work with research in medical literature to externally validate our analytics. Rare and orphan diseases often have online aggregating sites for collecting and connecting research and patients. This provides context for the prevalence of these rare genetic diseases.
Prime is well known for its monthly and quarterly pipeline updates, published on our website. We also provide proprietary drug watch list information to clients.
The pipeline research adds a lot of information that we build into our integrated medical and pharmacy analytics. All of this helps us identify potential patients that could be treated with a gene therapy once it is approved.
Once we can identify what patients might use this drug therapy, we can calculate the average PMPM impact. More importantly, we are able to provide plan-specific PMPM. We provide all of that information today to our plans through Insights+.
- For costs, we do plan-level analytics to help identify individuals who might be potential patients for gene therapies.
- For timing, we help plans understand when these costs are coming.
- For performance, we pursue and prioritize value-based contracts in the gene therapy space.
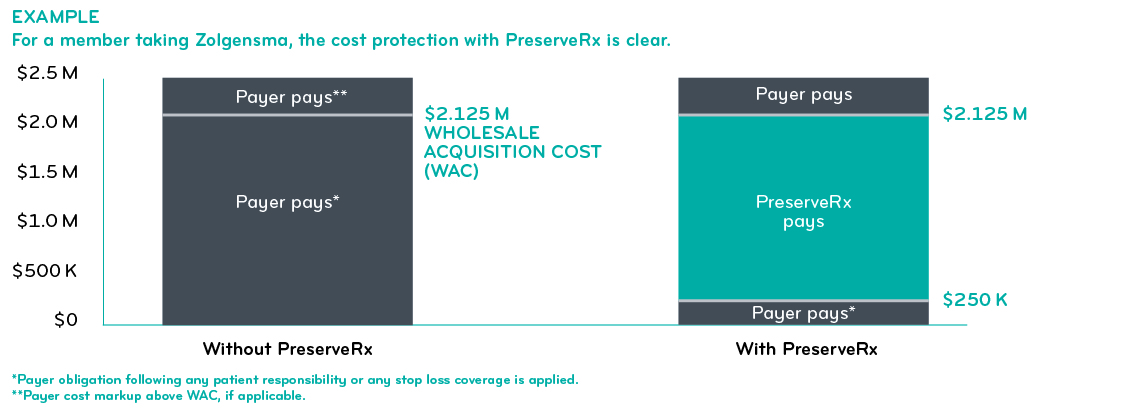
What is PreserveRx?
In 2020, Prime and BCS Insurance Company introduced PreserveRxSM, its gene therapy reinsurance solution. PreserveRx is an excess reinsurance product with configurable attachment points for health plans. It covers gene therapies at 100% of WAC. Pricing is set at a per member per month premium.
Prime offers PreserveRx along with BCS Financial, which is wholly owned by all Blue Plans. Prime provides the analytics around probabilities and likelihoods of gene therapy potential candidates and uptake. BCS Financial does the actual underwriting for a given population.
PreserveRx is currently available only to commercial customers.
Prime offers follow-on value-based contracts for gene therapy products limited to the contract terms with the pharmaceutical manufacturer.
How does PreserveRx support plans, employers and members?
PreserveRx helps health plans reduce financial risk in a changing gene therapy market. PreserveRx covers current FDA-approved gene therapy drugs, Luxturna® and Zolgensma®. As more high cost genetic therapies are approved by the FDA, the coverage will be expanded, and pricing will be updated. The focus is on drugs that treat rare genetic conditions with a one-time treatment.
- Robust forecasting, underwriting and cost impact analysis
- Detailed data and advanced expertise to make sure members get the right treatment
- Eligible and earned rebate savings go to the plan
- Enhanced access, outcomes tracking and reporting
Prime’s total drug management works to balance breakthrough treatments like gene therapy with responsible and careful cost management. We analyze fully integrated medical and pharmacy data to understand the entire scope of a patient’s health. Prime continues to provide actionable data to help our clients look at issues of both affordability and accessibility.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts