Perspectives
Palforzia is only drug approved to treat peanut allergy in children
Peanut avoidance remains the first and best defense
January 7, 2020Drug name: Palforzia™ (AR101)
Manufacturer: Aimmune Therapeutics
Condition: Peanut allergy
PDUFA: 1/2020
Drug name: Viaskin™ Peanut
Manufacturer: DBV Technologies
Condition: Peanut allergy
Condition overview
Peanuts are the second most common food allergy in children and it’s on the rise.1 Roughly 1.2 million children and teens in the United States — or just over 2% of the non-adult population — have peanut allergies.1 Although most life-threatening reactions are triggered by ingested foods, reactions can also come simply from skin contact, eye contact or smelling peanuts. About 20 percent of children outgrow peanut allergies. But those with one nut allergy have a 1:4 chance of being allergic to other nuts.2
Current treatments and cautions
Currently, the best treatment is total avoidance. This means:
- Keep no peanut products on the premises because of other food contamination
- Carry a supply of safe food when travelling
- Learn to read labels carefully
- Be aware that other nut products may present a risk
- Tell family, caterers, and party hosts about the allergy well in advance of any gathering
- Always carry an EpiPen®
- Educate family members and caregivers about the signs of an anaphylactic reaction; teach them how to use an EpiPen
- Wear a medic alert bracelet
New guidelines suggest introducing infants to peanuts between four and six months of age. This may make them less likely to develop peanut allergies than waiting until after the infant is 12 months of age.3
Overview
The FDA is expected to approve oral immunotherapy (OIT) products Palforzia (AR101) in 4Q2019 or 1Q2020, and Viaskin Peanut in 2020.
The peanut desensitizers may increase patient tolerance to peanuts; in clinical trials, patients who couldn’t tolerate a 1/3 of a peanut kernel could tolerate 2 to 4 peanut kernels after treatment with a peanut desensitizer. However, the risk of a systemic allergic reaction was higher in the treatment group than in the placebo group.
ICER concluded that neither of the peanut desensitizing agents offer a superior net health benefit compared to peanut avoidance.4
How effective are Palforzia (AR101), Viaskin and peanut oral immunotherapy (OIT) compared to no treatment?4
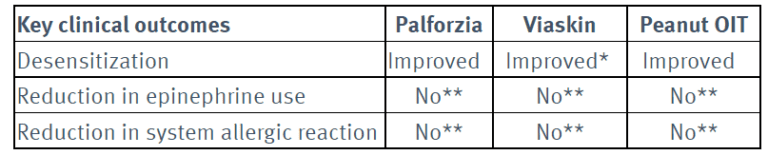
*A greater proportion of patients treated with Viaskin achieved desensitization compared to the no treatment arm, however the predefined primary outcome of the trial was not met.
** In studies of Palforzia (AR101), Viaskin, peanut OIT, epinephrine use and systemic allergic reactions increased.
Drug name: Palforzia™ (AR101)
Condition: Peanut allergy in children and adolescents 4-17 years old
Benefit: Pharmacy and medical benefit as each dose increase must be observed by a health care professional
ROA: Administered as an oral powder in graduated doses in pull-apart capsules or foil-laminate sachets. The contents are mixed thoroughly with a few spoonfuls of food. Initial dose and first dose of each dose-escalation should be administered in a facility equipped to treat systemic allergic reactions.5
Drug name: Viaskin™ Peanut
Condition: Peanut allergy in children and adolescents 4-11 years old
Benefit: Pharmacy benefit, first application must be observed by a health care professional
ROA: Non-invasive, once-daily epicutaneous patch6
Prime monitors the drug pipeline
The drug pipeline is full of new, groundbreaking specialty drugs that may help members feel better and live well. The drug watch list is just one of Prime’s clinical strategies designed to keep clients ahead of drug trends — because it’s easier to manage change when you see it coming.
References
- https://www.medpagetoday.com/meetingcoverage/acaai/76459
- https://www.slhd.nsw.gov.au/rpa/allergy/resources/allergy/peanutallergy.pdf
- https://www.aaaai.org/about-aaaai/newsroom/news-releases/early-peanut-introduction
- https://icer-review.org/wp-content/uploads/2019/07/ICER_PeanutAllergy_RAAG_071019.pdf
- http://ir.aimmune.com/news-releases/news-release-details/fda-allergenic-products-advisory-committee-votes-support-use
- mag.com/medical-news/fda-bla-viaskin-peanut
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts