Perspectives
Helping patients with severe asthma breathe easier
Finding ways to help new drugs get used by those who can benefit from them.
November 6, 2018I’m fascinated by the mechanism of action of drugs,” said Mary Claire. “Science can help us understand how the body responds to different triggers. This helps us find the patients who will benefit the most from certain drugs.”
New biologic drugs for advanced asthma are expensive. And these costs are rising. “So we find new and better ways to make sure that drugs are used by the most appropriate patients – those who will benefit from these effective but expensive therapies,” explained Mary Claire.
Advanced care for severe asthma
Asthma is a chronic inflammatory disease of the airways. It is characterized by patterns of symptoms, such as wheezing, coughing, chest tightness and shortness of breath. According to the Centers for Disease Control and Prevention (CDC), 26 million people in the United States (1 in 13) have asthma. About 30 percent of this population have intermittent symptoms and 70 percent have persistent symptoms. Of those with persistent symptoms, about 5 percent are considered to have severe asthma.
Severe asthma doesn’t respond as well to common controller medications, such as inhaled corticosteroids. People with severe asthma may require high doses of inhaled corticosteroids or even oral corticosteroids. Even then, they may still not find relief. This population is most at risk for hospitalization and death due to asthma.
There’s a lot we don’t know about severe asthma
“I’ve studied asthma for years and there’s still a lot we don’t understand about the disease process,” said Mary Claire, “But scientific research is finally starting to provide some answers to questions about the causes of inflammation at the cellular level. We hope this continued research will help find solutions to keep patients with asthma out of the hospital.”
Some patients have asthma that is exacerbated by environmental factors, such as pet allergies or exposure to mold. Others have asthma without obvious triggers. Part of the definition of the disease is that patients’ symptoms vary over time. A patient may experience symptoms that are so severe they require hospitalization at one time, but just have moderate symptoms later.
 An eosinophil is a type of disease-fighting white blood cell. Elevated eosinophil levels are a sign of more severe asthma.
An eosinophil is a type of disease-fighting white blood cell. Elevated eosinophil levels are a sign of more severe asthma.
“Asthma is classified by symptom severity,” Mary Claire explained. “But from a treatment perspective, we look to specific biological markers. In simple terms, as levels of these biomarkers go up, for some patients it indicates a more severe asthma type, where symptoms are harder to control. If a drug treatment works on a particular biomarker, the level goes down and symptoms improve. Now that we have drugs that work in different ways against different biomarkers, we can tailor therapy to provide better asthma control.”
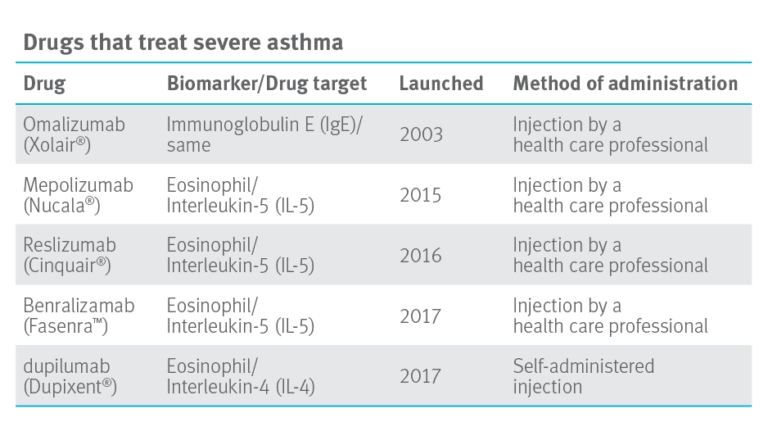
The drugs in the table below are effective at reducing asthma flare-ups and reducing the need for oral corticosteroids in patients dependent on corticosteroids to control inflammation in their lungs. Mary Claire explained, “Having more options is good for patients with severe asthma. However, these biologic drugs are expensive. Costs run from $2,600 to $4,800 per month.”
The Institute for Clinical and Economic Review (ICER) publishes studies on the clinical effectiveness and value of drugs. Its recent report on advanced asthma reports that “These medications all modestly reduce exacerbations of the disease,” The findings go on to read: “with the evidence available at this time, these biological agents seem to be priced higher than the modeled benefits over a lifetime at commonly accepted cost-effectiveness thresholds.”1 At the current price points, getting the right drug to the right patient becomes even more important.
Prime’s recommendation for health plan clients
Mary Claire presented an overview of the drug treatment outlook for severe asthma at a meeting of Blue Plan pharmacy and medical directors in June 2018. Per member per month (PMPM) costs have doubled in two years, due largely to drugs billed under the medical benefit. Based on Prime’s research, Prime’s strategy recommendation includes a combination of utilization management tools and a rebate strategy.
To guide appropriate use, Prime recommends a utilization management program that asks for:
- Optimization of first line therapies (e.g., inhaled corticosteroid) and
- Identification of specific biomarkers (IgE, eosinophils) to guide selection of appropriate drug
When patients meet these criteria, the right drug can be prescribed for the right patient and expensive drugs are not used in patients who will have little to no benefit.
It’s also important to have a medical policy in place so that drugs are billed under only one benefit set: either pharmacy or medical. Prime develops robust medical policies that our health plans clients may adopt.
Longer-term, there may be more options
“Looking ahead, there are additional drugs in the pipeline that, if approved by the FDA, will give patients with asthma new options, including an oral (i.e., pill) option,” Mary Clare said. “If these drugs make it to market, it will be in 2020 or after. “We don’t expect pricing to be reduced with these new drug entrants. We will continue to need to apply management strategies so that these drugs are being used for the most appropriate patients.”
“We are also looking at partnering with manufacturers for value-based contracts. This will provide a closer link between patient outcomes and the drug being used. It helps us provide coverage of these new drugs for the patients who need them,” Mary Claire concluded.
Plus, it’s good science.
References
1. Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectivenewss, Value, and Value-Based Price Benchmarks. Evidence Report published Nov. 13, 2018 by ICER. © The Institute for Clinical and Economic Review, 2018. Accessed at: https://icer-review.org/wp-content/uploads/2018/04/Asthma-Revised-Report-FOR-PUBLICATION-11.13.2018.pdf
Drug names are the property of their respective owners.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts