Perspectives
April Fraud Focus: How to identify counterfeit GLP-1 weight-loss drugs
April 9, 2024Last week, we kicked off our April Fraud Focus with a roundup of some of the latest fraud trends. Chief among them were schemes related to glucagon-like peptide 1 (GLP-1) agonists, a class of drug often used for weight loss.
As we noted earlier this year, the demand for GLP-1 drugs continues to rise, in fact, J.P. Morgan Research predicts that by 2030, about 9% of the U.S. population will use GLP-1s. And while we have seen GLP-1 shortages rebound, the popularity of these drugs means that fraud will also follow the trend.
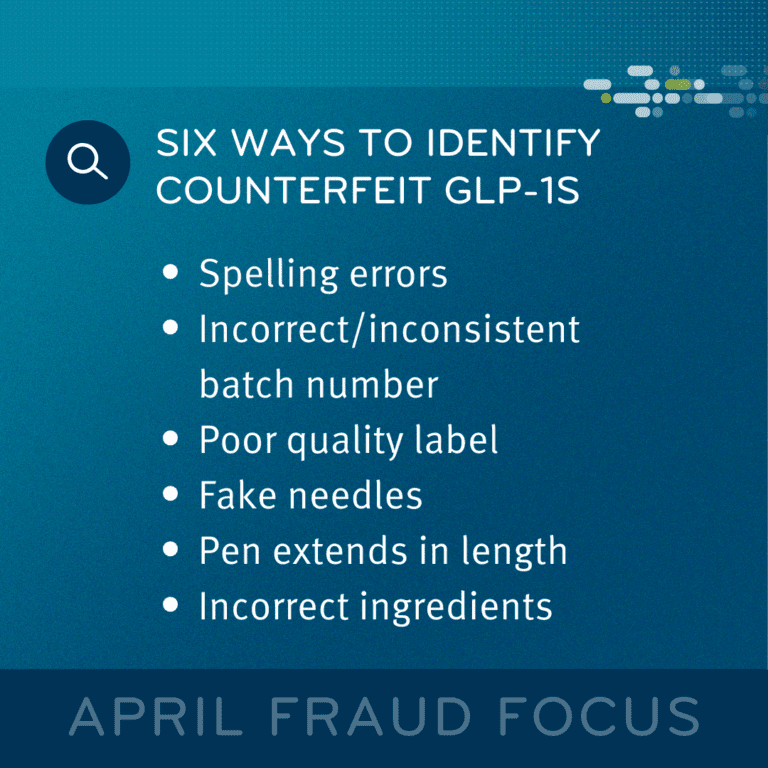
As the balance of supply and demand continues to be tested, industry experts caution consumers to know where they are getting their drug supplies. As is often the case when a new, popular class of drug hits the market, we see a rise in cases where bad actors — particularly using online of app-based platforms — supply consumers with counterfeit, unapproved, misbranded, and/or contaminated drugs. Worse still, some counterfeit drugs may contain ingredients used to stretch the active ingredients, and/or be improperly stored and transported, which may render them unsafe or ineffective.
We also addressed counterfeit drugs earlier this year, including tips to keep you safe as the FDA continues to investigate illegal claims.
And while the safety of these supplies should be concerning enough, beware that in most fraud, bad actors are more interested in the financial gains inherent in their schemes. We continue to hear reports of fraudulent suppliers offering pharmacies unapproved GLP-1s at a discount, accepting full or partial payment, only to turn around and not deliver the product to the pharmacies. Or they may deliver faulty products that are missing tamper-resistant measures or mixing and matching applicators with different boxes/stated doses. What’s more, we’ve heard reports where the included needles may be fake, or worse still, unsterile.
At least three U.S. residents were hospitalized last year after using an allegedly counterfeit version of Ozempic. While there have been no reports of counterfeit Wegovy pens in the U.S., experts still recommend caution, encouraging retail pharmacies and consumers only to purchase authentic therapies through authorized distributors.
The Prime Therapeutics/Magellan Rx (Prime/MRx) Special Investigations Unit (SIU) works to educate pharmacies and health care leaders about potential schemes and associated risks to patients through regular communications to network pharmacies.
As of today, we have not identified counterfeit GLP-1 supplies in our investigations, but we’ve created new analytical models to identify potential hospitalizations after a member has filled a GLP-1 prescription to monitor for complications. This is a common practice when it comes to certain drug classes as it’s a frontline defense in tracing and identifying potential counterfeit drug supplies.
Prime/MRx also recently launched a new GLP-1 Strategy page, which compiles all our GLP-1 resources and thought leadership, including research, podcasts, videos, recent articles and more. We take pride in utilizing an evidence-based approach to GLP-1 drugs, working with our partners to develop individualized strategies that promote clinically appropriate use and consider total cost of care, long-term value and health outcomes.
Stay tuned for more fraud tips next week, and watch for more information on X @Prime_PBM.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts