Perspectives
Analysis of 74 CAR-T therapy patients inform a Blue + Prime future
We’re hitting the cell and gene therapy learning curve with everything we’ve got.
July 23, 2021Cell and gene therapies have been available for several years, but they are still rare and very expensive. Most employers don’t have any direct experience with them — yet.
So, here’s real-world data you can sink your teeth into. Real patients, real numbers.
The analysis presented here comes from our:
- Study of 74 patients taking a chimeric antigen receptor T-cell (CAR-T) therapy: either Yescarta® (axicabtagene ciloleucel) or Kymriah® (tisagenlecleucel)
- Expertise with health outcomes research, enriched with decades of proficiency with integrating medical and pharmacy claims
- Long-term relationships with Blue Plan clients across the country
- Experience forecasting utilization of high cost drug therapies
The growth of cell and gene therapies is making big changes to our health care system. There’s a lot we don’t know. So, let’s start with what we do know.
Our study showed that CAR-T costs are only part of the big picture
The process of CAR-T therapy is complex. It involves extracting a patient’s T-cells, and shipping them to a facility where they are genetically reprogrammed to fight the patient’s cancer. Before the new T-cells can be infused, the patient must undergo conditioning chemotherapy to create a favorable environment. Then, following administration, patients must be monitored for adverse reactions such as cytokine release syndrome and neurological toxicities. The average hospital stay for patients in the study was 17 days.
From its 15 million commercially insured member population, Prime identified 74 patients who were treated with Yescarta® or Kymriah® between January 2018 and June 2020.
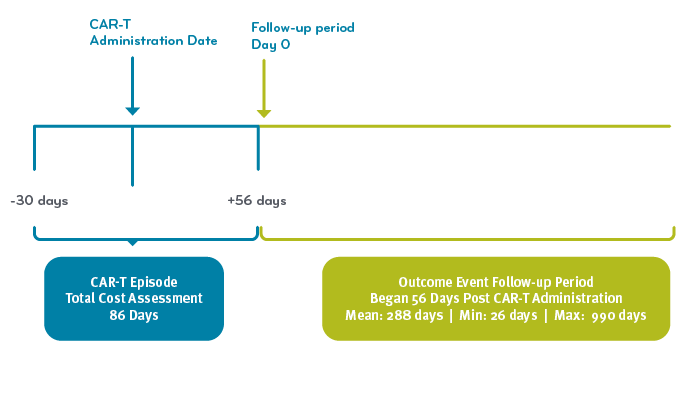
CAR-T Episode and Follow-up Analysis Timeline
We defined a CAR-T episode as 86 days: 30 days prior to, and 56 days after the CAR-T administration. Then, we found that 40% of patients went on to have a clinical event several months after CAR-T administration (defined as additional chemotherapy, a bone marrow transplant, or death). That meant the results were not as durable as we’d hoped.
For that reason, we examined CAR-T care in three parts:
- CAR-T drug costs
- Episode costs surrounding drug administration; episode costs include any additional medical or pharmacy charges that fell within the 86-day time period
- Post-episode results, including clinical events and outcomes in the months following the 86-day CAR-T episode
Hospitals added varying mark-ups to CAR-T administration
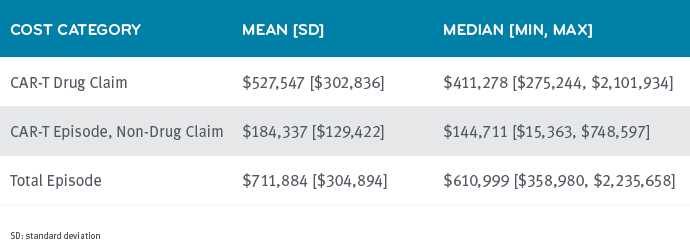
The wholesale acquisition cost (WAC) of CAR-T-cell therapies is $373,000. The mean cost of the CAR-T drug across the study was $527,547. One plan paid more than $2 million for one CAR-T administration. (This seems like an unreasonable mark-up, but that’s as much as we could see based on claims.)
In all 74 cases analyzed, the CAR-T administration was paid under the medical benefit through contracts held between the payer and hospital. Drugs paid through the medical benefit are frequently priced higher than the WAC. We expected that, based on Prime’s experience with other medical drugs, but this broad range was still surprising.
This is one of the reasons Prime developed MedDrive™. This drug management program helps health plans identify and design medical policies and reimbursement contracts that can help make mark-ups more consistent and transparent.
The CAR-T WAC is consistent, but total insurer payments showed substantial differences. The average mark-up over WAC was $154,000 on each CAR-T claim, but went as high as $1.73 million over WAC. The wide variation in payer CAR-T administration costs might provide a cost management opportunity.
Patients can have varied responses to gene therapy
The episode costs surrounding drug administration also showed a wide range. That’s because patients vary in their response to therapy, like side effect management costs, e.g., cytokine release syndrome, or hospital stay length. In fact, the range here went from $15,363 to $748,597.
Total costs of treatment
Average cost of care for the total episode was $711,884, but 12% of patients had care that resulted in costs of more than $1 million.
CAR-T B-cell Lymphoma Episode Costs among 74 Members
Are these patients better? Did they get value from the treatment?
These are tough questions. Because of how much we’re learning about cell and gene therapy, there’s much we don’t know. But that doesn’t mean we don’t ask questions and look at the data.
First, who received these treatments? The 74 patients were 18 to 76 years old. The average age was 55. They were 60% men. They’d already had relapses of their cancer. (CAR-T therapies are indicated only for people who have already had at least two forms of systemic therapy.)
Prime followed patients beyond the 86-day CAR-T episode
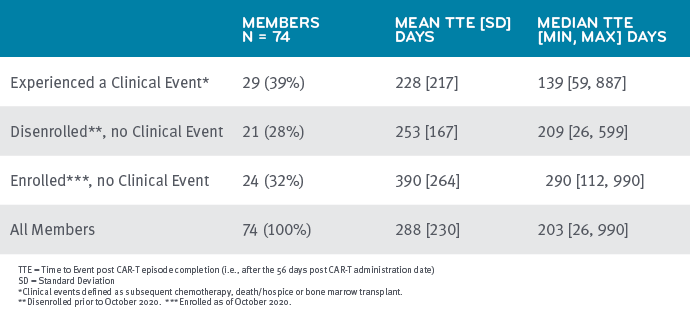
We followed the CAR-T patients until the end of 2020 or until they had disenrolled, whichever came first. This time period began at the end of their 86-day CAR-T episode. We were able to follow the 74 patients for an average of nine months.
During the follow-up period, the CAR-T therapy was successful for about one-third of the patients. These patients showed no further claims for cancer treatment during this time. But one in three patients received additional cancer treatment — primarily chemotherapy, but also bone marrow transplants — during the follow-up period. We can also identify deaths that are assigned a certain discharge status code. So we know that 13 of 74 patients died (18%) at an average time of about a year after their CAR-T therapy.
We lost visibility to another one quarter of the study participants. They disenrolled. This percentage seems high. Knowing what happened to them could have a significant impact on the study. We would need to compare this to oncology drug utilization in general to see if this is typical attrition.
The study is limited to claims-based information. For patients that leave their plans, the study data could be missing clinical events.
Status of 74 Members Receiving CAR-T Therapy for B-cell Lymphoma
Prime’s data provides context for a price-to-value discussion on CAR-T therapy
A 2018 Institute for Clinical and Economic Review (ICER) report on the two CAR-T therapies suggested they are in alignment with clinical value despite their high price. The assessment also noted concerns around long-term risks and benefits.
ICER forecasts that current CAR-T costs will be difficult for the health care system to absorb without shifts in payment models, making access a problem for patients.¹
 Prime’s study provides real world evidence of those risks, noting 40% of patients did not have a durable CAR-T response. Little has been published to date about the costs of these post-CAR-T clinical events that treat patients with B-cell cancers.
Prime’s study provides real world evidence of those risks, noting 40% of patients did not have a durable CAR-T response. Little has been published to date about the costs of these post-CAR-T clinical events that treat patients with B-cell cancers.
Analysis like this can help inform performance metrics and value-based contracting with providers and pharmaceutical manufacturers. In addition, given the pipeline potential, it also helps inform forecasting for CAR-T’s increased volume of treated patients and spend.
Outcomes for members with cancer are steadily improving, but the system-wide cost of this improved care is dramatically increasing.² These findings help Prime and our health plans clients understand real-world CAR-T therapy cost of care. It helps quantify the value members receive over time from prescribed treatments. We use these insights to provide data-driven recommendations to our health plan clients and prescribers.
References
- Institute for Clinical and Economic Review Final Report on CAR-T Therapies Highlights Need for Systemwide Changes to Ensure Affordability and Access to Innovative, One-Time Therapies.” March 18, 2021. By Staff. ICER. Accessed at: https://icer.org/news-insights/press-releases/car-t-final-report/
- Polite BN, Ratain, MJ, Lichter AS. Oncology’s “Hockey Stick” Moment for the Cost of Cancer Drugs-The Climate Is About to Change. JAMA Oncology 2021;7(1):25-26. https://jamanetwork.com/journals/jamaoncology/article-abstract/2771013
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts