Perspectives
A deep dive into cost of care for rheumatoid arthritis (RA)
A look at real-world RA cost of care and treatment compared to guidelines
July 31, 2018What were the studies about?
Up to 23.5 million Americans suffer from autoimmune diseases, which includes rheumatoid arthritis.1 According to the National Institutes of Health, the prevalence is rising. Within Prime’s commercial book of business in 2017, biologic anti-inflammatory treatments led spending among specialty categories, comprising more than 14 percent of total expenditures.2
These two studies looked at the cost of care for RA and the biologic drugs that treat it. By analyzing integrated medical and pharmacy claims, the studies compared real-world treatment patterns with recommended clinical guidelines.
What did we learn?
Members with RA had 3.5 times higher total cost of care than members without RA. Members with RA taking biologic/targeted synthetic DMARDs (b/tsDMARDs) had 3 times higher total cost of care than members taking conventional DMARDs (csDMARDs).
Cost comparison of member not on specialty drugs to member with RA on DMARDs Cost comparison of member not on specialty drugs
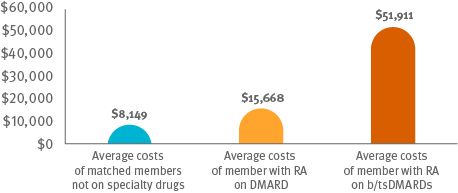
RA: rheumatoid arthritis; DMARD: disease modifying anti-rheumatic drug; csDMARD: conventional synthetic DMARD; b/tsDMARD: biologic/targeted synthetic DMARD.
Methods
For both studies, Prime looked at continuously enrolled commercially insured members from 2013 to 2016, younger than 65. Of 3.5 million members that met these criteria; 26,098 (0.7 percent) had RA. From the remaining members, Prime selected matched non-RA members not taking specialty drugs for comparison purposes.
Results
Based on analysis of integrated medical and pharmacy claims, it appears that the use of DMARDs does not always follow national clinical guidelines.
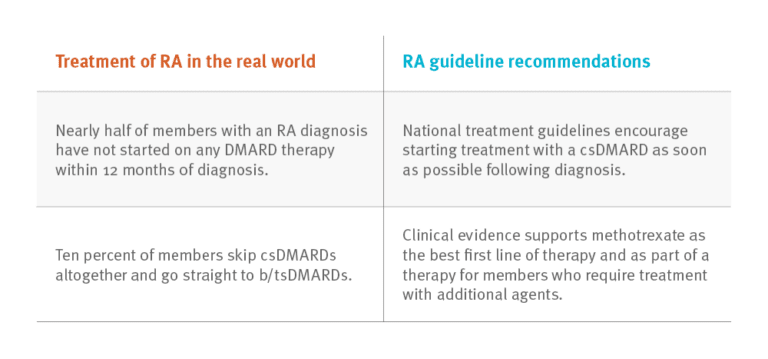 Nearly half of members with an RA diagnosis have not started on any DMARD therapy within 12 months of diagnosis. National treatment guidelines encourage starting treatment with a csDMARD as soon as possible following diagnosis.
Nearly half of members with an RA diagnosis have not started on any DMARD therapy within 12 months of diagnosis. National treatment guidelines encourage starting treatment with a csDMARD as soon as possible following diagnosis.
Ten percent of members skip csDMARDs altogether and go straight to b/tsDMARDs. Clinical evidence supports methotrexate as the best first line of therapy and as part of a therapy for members who require treatment with additional agents.
Conclusions
RA guidelines supporting use of csDMARDs before b/tsDMARDS have been in place for five years. The data show a lot of opportunity for broader use of csDMARDs before proceeding to b/tsDMARDs. Optimizing csDMARD use has the potential to lower the total cost of care in this category.
Additionally, the research found suboptimal use of triple csDMARD therapy* and high biologic DMARD discontinuation rates in RA patients. Again, optimizing csDMARD use could lower the total cost of care in this category. Decreasing the use of b/tsDMARDs and more closely monitoring its use could reduce waste, which adds cost to the health care system.
What does this mean for you?
Utilization management strategies can be designed to encourage compliance with RA guideline recommendations and use of the most cost-effective lines of therapy.
Managed care interventions could address the apparent gap in care for the 40 percent of members with a diagnosis of RA but no DMARD therapy start in their first year.
Prime’s GuidedHealth® program provides this kind of actionable clinical intelligence to doctors, members and plans. These insights can result in improved care, safer medicine use, better outcomes and lower overall cost of care.
* Triple combination DMARD therapy refers to the use of three csDMARDs before considering a move to b/tsDMARD. A significant proportion of patients achieve sustained remission with triple therapy and do not progress to b/tsDMARDs.3-7
References
- https://www.aarda.org/news-information/statistics/
- Prime Therapeutics. Focus on Trend: Commercial. Spring 2018.
- Jalal H, O’Dell JR, Bridges SL, et al. Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthritis Care Res 2016; 68(12)):1751-1757.
- Bansback N, Phibbs CS, Sun H, et al. Triple therapy versus biologic therapy for active rheumatoid arthritis: A cost-effectiveness analysis. Ann Int Med 2017;167:8-16.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. NEJM 2014;371:396-397.
- Hazlewood GS, Barnabe C, Tomlinson G, et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 2016;353:I 777.
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004; 364:263-269.
GuidedHealth is a registered trademark of Prime Therapeutics LLC.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts