Perspectives
Real world data on Harvoni® cure rates
October 1, 2017Prime data shows a 97% hepatitis C cure rate based on test results
Harvoni, as a cure for hepatitis C, has been on the market since 2013. But not much real-world data has focused on laboratory cure confirmation results (i.e., achieving sustained virologic response) along with a treatment persistence assessment. This study does.
What was the study about?
Hepatitis C treatment is expensive and complex. It can have severe side effects. It requires commitment from the member to start, follow and complete the therapy. This study analyzed treatment completion/cure rates, as measured by sustained virologic response (SVR) in laboratory results. The SVR is used to determine whether the treatment response — the cure — is sustained weeks after treatment is complete.
What did we learn?
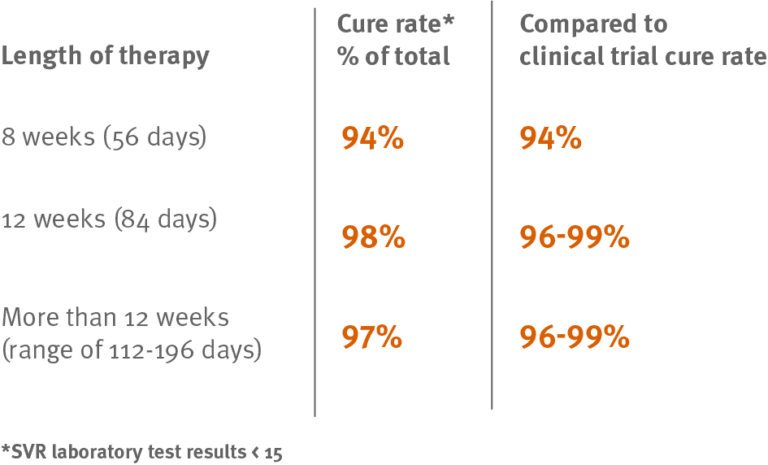
Nearly all the commercially insured members (97 percent) who completed at least eight weeks of hepatitis C treatment with Gilead’s Harvoni® (ledipasvir/sofosbuvir) were cured of the disease. The results are similar to clinical trial data, which found cure rates of 94 to 99 percent.
Methods
For the study, Prime researchers reviewed data from 311 commercially insured individuals who received at least eight weeks of Harvoni therapy and provided a sustained virologic response (SVR) test result to the pharmacy between 12 to 24 weeks following Harvoni use.
Results
Researchers found 301 of 311 members (97 percent) had a SVR laboratory result of 15 or less, indicating a cure. ¹
SVR rates by weeks of therapy
Conclusions
The study group also received additional care management services from Prime Therapeutics Specialty Pharmacy.1 This included 24-hour, 7-day access to experts who closely managed the coordination of pharmacy and medical benefits and helped members follow their treatment plans. This support is associated with higher levels of adherence.
What does this mean for you?
This real world data analysis found members treated for hepatitis C virus with Harvoni through the Prime Therapeutics Specialty Pharmacy had cure rates equivalent to those reported in the clinical trial data. This finding supports both Harvoni real world effectiveness and the value of using a specialty pharmacy for hepatitis C treatment.
Plan sponsors should make sure all members taking specialty medicines have the care management services of an accredited specialty pharmacy supporting them throughout their treatment plans.
References
- At the time of this research, Prime Therapeutics Specialty Pharmacy (Prime Specialty Pharmacy) was a wholly owned subsidiary of Prime Therapeutics LLC. On April 1, 2017, Prime Therapeutics Specialty Pharmacy became part of a new company formed by Prime Therapeutics LLC and Walgreen Co., headquartered in Orlando, FL. The new company, AllianceRx Walgreens Prime, has the capacity, geographic distribution and operational efficiencies to serve large health plans and national employer groups. Prime Therapeutics retains an ownership interest in AllianceRx Walgreens Prime.
Drug names are the property of their respective owners.
Related news
Perspectives
July 25, 2024
Quarterly Drug Pipeline: July 2024
Clinical insights and competitive intelligence on anticipated drugs in development
Perspectives
July 22, 2024
Oncology Insights: 2024 ASCO Annual Meeting key findings
Findings from this year’s American Society of Clinical Oncology (ASCO) Annual Meeting will likely lead to clinical practice changes and U.S. Food and Drug Administration (FDA) drug approvals or expansions
Perspectives
July 16, 2024
LISTEN NOW: Beyond the business – Stories of corporate kindness | Pharmacy Friends Podcast
In this episode, we talk about how our employees' help goes beyond our work in health care, aiding in philanthropic efforts