Perspectives
Cost of care for six months of preventive HAE therapy: $335,000 or more
Analysis of two preventive HAE drugs show they are overpriced, cost to value
January 21, 2020Real-world integrated claims data from use of two preventive drugs for hereditary angioedema (HAE) show they are overpriced, cost to value¹
Two HAE preventive therapies came on the market since Prime’s HAE study in 2018:
- Haegarda (C1-INH), launched June 22, 2017 and
- Takhzyro (lanadelumab), launched August 23, 2018.
This gives members with HAE three treatment options for preventive care. (Cinzyre (C1-INH) is the third, and not part of this study.)
HAE is a extremely rare and potentially life-threatening genetic condition. Patients often use a combination of on-demand treatment and long-term preventive treatment.
Prime identified 58 members that qualified for the study criteria, out of its 15 million commercial members. To assess total cost of care, the study looked at integrated medical and pharmacy data for these members and their use of one of the two preventive care drugs. It also tracked their use and cost of additional on-demand HAE treatment drug therapy.
Comparison of six-month mean costs
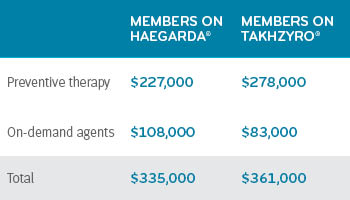 Haegarda’s mean cost for six months was nearly $227,000. Takhzyro mean cost for six months was more than $278,000. That’s a difference of $51,000 (22%) for six months of treatment. Members taking Haegarda had a total six-month mean cost of $335,000 (which included additional on demand therapy and their Haegarda therapy). Takhzyro members had a total six-month mean cost of $361,000 (which included additional on-demand therapy plus their Takhzyro therapy).
Haegarda’s mean cost for six months was nearly $227,000. Takhzyro mean cost for six months was more than $278,000. That’s a difference of $51,000 (22%) for six months of treatment. Members taking Haegarda had a total six-month mean cost of $335,000 (which included additional on demand therapy and their Haegarda therapy). Takhzyro members had a total six-month mean cost of $361,000 (which included additional on-demand therapy plus their Takhzyro therapy).
A report from the Institute for Clinical and Economic Review indicates these drugs exceed thresholds for cost-effectiveness.¹
Prime’s proficiency in data analytics turns integrated benefits claims data into actionable, real world cost of care information. This can help inform preferred drug placement decision making and value-based contract negotiations with pharmaceutical manufacturers.
Reference
1. Institute for Clinical and Economic Review Issues Final Report on Long-Term Prophylaxis for Hereditary Angioedema, Provides Policy Recommendations to Improve Cost-Effectiveness. Nov. 15, 2018. Boston. © ICER, 2021. Accessed at: https://icer.org/news-insights/press-releases/institute-for-clinical-and-economic-review-issues-final-report-on-long-term-prophylaxis-for-hereditary-angioedema-provides-policy-recommendations-to-improve-cost-effectiveness/
Related news
Perspectives
April 25, 2024
Clinical News: April 2024
Your monthly source for drug information highlights
Perspectives
April 25, 2024
Drug Approvals Monthly Update: April 2024
This monthly update of United States (U.S.) Food and Drug Administration (FDA) approvals…
Perspectives
April 25, 2024
Drug Approvals Quarterly Update: April 2024
This wrap-up provides a review of newly approved drugs, recent drug launches, new…