Perspectives
Targeted gene therapies are revolutionizing cancer treatment
Can we transform health care delivery to keep up?
January 8, 2021Just a few years ago, before I came to Prime, I had a patient come to me who was very ill. A PET scan showed advanced cancer in a massively enlarged liver and cancer in his bones. Results from a liver biopsy were classified as an adenocarcinoma. But in the cancer field, that term is very generic. it’s like saying “dog.” You can’t tell if you’re seeing a Great Dane or a Chihuahua. In this case, the cancer was so far spread, we couldn’t even tell where it first started.
The best way to proceed was to do gene sequencing to try to understand the cancer’s DNA blueprint. But at that time, insurance companies were not approving these requests. We identified a clinical trial that would allow us to complete the sequencing for free, but it was going to take time. While waiting for the sequencing results, we started him on chemotherapy, but it was not going well. I told the family he only had a matter of months left to live.
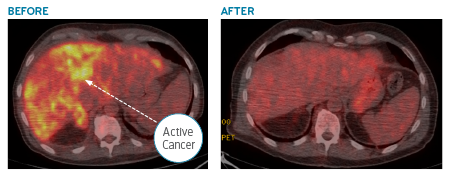 When we got the sequencing back, it showed the cancer’s origin was in the lungs. From the lungs, it had spread to the liver and bones. The sequencing showed that the cancer’s DNA had a mutation called ALK.
When we got the sequencing back, it showed the cancer’s origin was in the lungs. From the lungs, it had spread to the liver and bones. The sequencing showed that the cancer’s DNA had a mutation called ALK.
There was an oral drug available that was FDA-approved to attack the ALK mutation. We immediately began treatment. Within days, the drug acted with laser-like precision, and the size of the cancer in the liver began decreasing and the patient started feeling better.
Every lock has a key
Lung cancers may look identical under a microscope, but they are very different on a genetic level. Over half of lung cancers have a genetic mutation responsible for allowing that cancer cell to replicate without limits. This unique genetic makeup allows us to use targeted drugs that are precise to that specific cancer.
Finding the right key (treatment) to the lock (cancer) can produce dramatic, life-saving results. DNA sequencing is a critical part of this process, because it allows the patient’s’ care team to tailor treatments for that specific cancer rather than trying to use a one size fits all approach like chemotherapy.
To wrap it all up: My patient’s cancer, first identified in his liver and bones, actually started as lung cancer. The gene sequencing helped us match it to a targeted therapy. Without gene sequencing, I didn’t think the patient would have lived more than a couple months. With sequencing – and the right gene therapy – the cancer disappeared within two months and the patient went into remission. The outcome was truly a miracle of modern medicine.
Lung cancer: not just a smokers’ hazard
You don’t hear as much about lung cancer as you do diseases like breast cancer. This is part due to the stigma from the link with smoking. While smoking remains the most common cause of lung cancer, the reality is that currently the majority of patients diagnosed with lung cancer had previously quit smoking, and many never smoked at all.
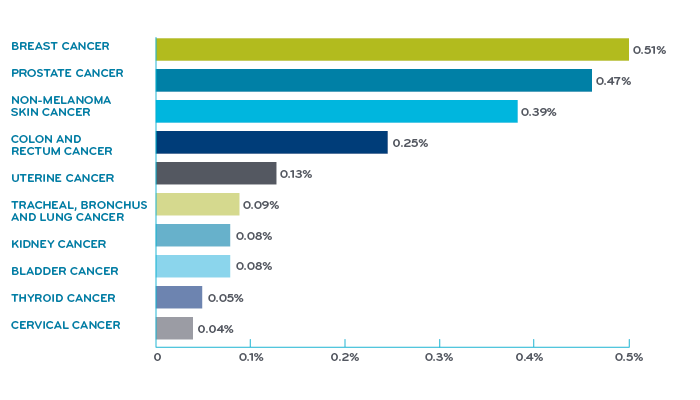
Cancer deaths by type, United States, 2017¹
Overall, lung cancer is the leading cause of cancer mortality. It causes one third of all cancer deaths. Breast cancer gets more visibility but lung cancer actually kills more women than breast and ovarian cancer combined.
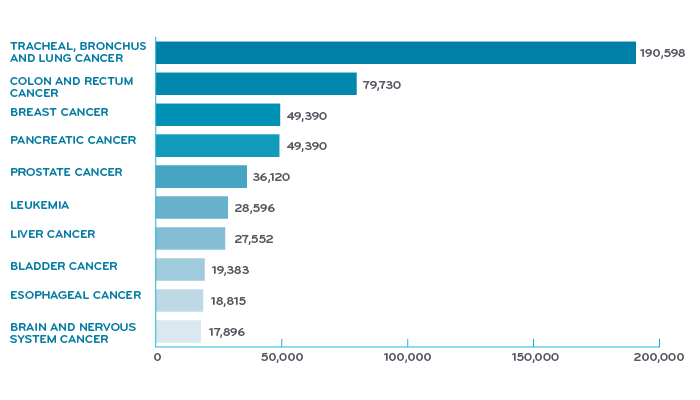
Share of population with cancer, United States, 2017¹
Breakthrough came from looking at the genetic blueprint of cancer
In 2005, investigators identified a mutation in a gene called EGFR. There was a drug developed that happened to work on that specific mutation. This was the first targeted drug approved for lung cancer. The understanding of the molecular biology of lung cancer exploded after that.
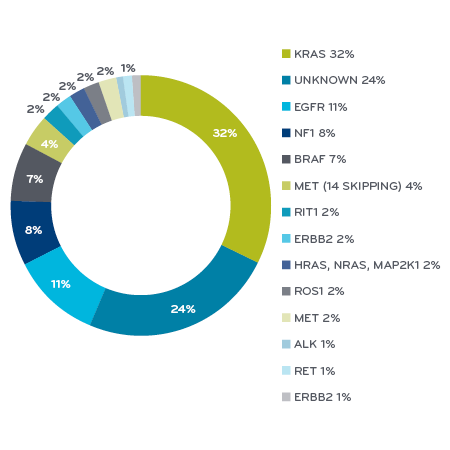
By 2009, scientists had identified separate genetic mutations that were present in over half of the most common type of lung cancer, shown by different colors in the pie. Every different color piece of the pie has at least one FDA-approved drug that can treat it or has a drug in development. For several of these mutations, we now have multiple approved drugs, with more on the way. This has completely changed the way the medical community thinks about how to treat lung cancers.
Genomic classification of non-small cell cancer subtypes²
 Understanding the use of these drugs across the health care system: When I came to Prime in April of 2020, I was pleased to see the kind of outcomes-based and total cost of care research Pat Gleason was producing with the Health Outcomes team using real-world integrated medical and pharmacy data. Last year, at the Academy of Managed Care Pharmacy, they presented a scientific poster on assessment of the real-world use of Tagrisso®, a growth factor receptor inhibitor (EGFR-i) total cost of care for the treatment of non-small cell lung cancer (NSCLC). (Tagrisso has an annual wholesale acquisition cost of $177,152.)
Understanding the use of these drugs across the health care system: When I came to Prime in April of 2020, I was pleased to see the kind of outcomes-based and total cost of care research Pat Gleason was producing with the Health Outcomes team using real-world integrated medical and pharmacy data. Last year, at the Academy of Managed Care Pharmacy, they presented a scientific poster on assessment of the real-world use of Tagrisso®, a growth factor receptor inhibitor (EGFR-i) total cost of care for the treatment of non-small cell lung cancer (NSCLC). (Tagrisso has an annual wholesale acquisition cost of $177,152.)
Tagrisso has been shown to be effective when used with patients who have the EGFR exon 21 or exon 19 mutation. (These are two of the most common mutations in non-small cell lung cancer patients.)3
The Prime real-world study showed that those taking Tagrisso had a 50 percent higher total cost of care than those using competitor products. Additionally, the study found that one in six members discontinued Tagrisso therapy during the first six months of use.4 This kind of real world data provides actionable information for pharmacy benefit design, member support and provider outreach programs, and a better understanding of the current real-world value that Tagrisso brings. (Prime’s study on Tagrisso earned a platinum ribbon at the fall 2019 AMCP meeting.)
This data provides us real-world Tagrisso utilization facts that can be shared with the pharmaceutical manufacturer to negotiate medication fair pricing to value. Prime negotiates market-leading value-based contracts with manufacturers, with a strong price-to-value model that aims to protect clients. In the case of Tagrisso, the manufacturer was unwilling to provide a discount, however, we continue in our efforts to obtain fair pricing to value.
Helping health care work better for cancer patients and providers
One of the reasons I was attracted to Prime was the opportunity to have a bigger impact on improving how we deliver health care. Prime’s mission is to make health care work better so that people can get the medicine they need to feel better and live well. It’s gratifying to see it brought to life in our programs and by my new colleagues. I’m in the right place.
For all patients, making health care work better is about managing that first mile of care by reducing friction throughout the drug approval and delivery process and reducing obstacles for patients.
In the case of lung cancer, making health care work better means working with our Blue Plan clients so that our patients and providers have easy access to important tools like gene sequencing. And when there is a drug match to the genome, reducing the obstacles for patients and providers with rapidly managed prior authorization. Making health care work better also means managing costs by establishing value-based contracts based on value pricing models with evidence-based care.
I don’t know exactly what the future will look like. I know it will include closer alliances across health care channels. I look forward to reshaping and revitalizing relationships across Prime, our Blue Plan clients and the doctors and providers we work with.
References
- Jemal, A, et al. (2017). Annual Report to the Nation on the Status of Cancer, 197-2014, featuring survival. JNCI: Journal of the National Cancer Institute, Volume 109, Issue 9, September 1, 2017. Accessed at: https://ourworldindata.org/cancer
- Evolving Cancer Classification in the Era of Personalized Medicine: A Primer for Radiologists, by Ailbhe C. O’Neill, MD, Jyothi P. Jagannathan, MD and Nikhil H. Ramaiya, MD. Copyright © 2017 The Korean Society of Radiology. January 2017 Korean Journal of Radiology 18(1):6 DOI: 10.3348/kjr.2017.18.1.6
- Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. By S.S. Ramalingam, J. Vansteenkiste, D. Planchard, et al. November 21, 2019. N Engl J Med 2020;382:41-50. DOI: 10.1056/NEJMoa1913662. Copyright © 2019 Massachusetts Medical Society. Accessed at: https://www.nejm.org/doi/full/10.1056/NEJMoa1913662
- J.J. Whalen, D.J. Eckwright, J.P. Burke, P.P. Gleason. Osimertinib First-Line Approval in Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Metastatic Non-Small Cell Lung Cancer (NSCLC) Impact on Utilization and Total Cost of Care among 15 Million Commercially Insured Members. Prime Therapeutics. AMCP, October 2019, National Harbor, MD. Accessed at: https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2019/document-amcpposter-lungcancer.pdf
Related news
Perspectives
April 19, 2024
AMCP 2024: Behind the Research with Prerak Parikh
Parikh, director of medical pharmacy strategy at Prime/MRx, shares the latest on interchangeable biosimilars
Perspectives
April 19, 2024
LISTEN NOW: Live at AMCP Annual 2024 – Digging into managed care pharmacy insights | Pharmacy Friends podcast
In this episode, Prime/MRx clinicians — along with special guests — discuss the hottest topics covered at the Academy of Managed Care Pharmacy (AMCP)'s 2024 Annual Meeting in New Orleans
Perspectives
April 17, 2024
AMCP 2024: Behind the research with YuQian Liu
Ahead of her session with Andy Killpack, Liu — senior director of specialty clinical solutions at Prime/MRx — shares current care management strategies for cell and gene therapy and the future of this exciting frontier