Perspectives
Small populations, big dollars, Part 2
Myasthenia gravis: Check the labeling. Thanks, but no thanks.
June 13, 2018Myasthenia gravis. Some specialty conditions are rare, challenging to treat and difficult to live with. And the annual drug therapy price tag for members for these conditions can top $1 million. With stakes this high, specialty drug management becomes even more important. Read about three of them here.
Part 1: Hemophilia. Part 2: Myasthenia gravis. Part 3: Hereditary Angioedema.
The U.S. Food and Drug Administration (FDA) has made it easier and faster to get breakthrough drugs to market. This can give people with rare conditions new hope and new treatments. But those of us in managed care still must pay attention to cost and appropriate use and give people with rare conditions our best judgment, based on the evidence.
Adding an indication to a drug already on the market
Eculizumab (Solaris®) has been on the market for more than ten years, approved to treat two ultra-rare conditions. The drug costs, on average, $704,000 per person for the first year.
The manufacturer applied to the FDA to treat people with a third rare condition – myasthenia gravis (MG), specifically, adult patients with MG who are anti-acetylcholine receptor (AchR) antibody positive. This blood protein (anti-AchR) is found in most people with MG.1
The clinical trial for the drug used a specific group of subjects: people with MG who were anti-AchR and who also were resistant to other MG therapies.
About 75 to 85 percent of people with MG are anti-AchR. Only about 10 percent of people with MG are resistant to other therapies.2
The drug was tested on a cross section of these two, which is a smaller group — people with MG who were anti-AchR and resistant to other therapies. But the FDA-approved label indication is for the larger group — people with MG who were anti-AchR.1
Prime recommends prescribing the drug only for those for whom it was tested – the clinical trial of people with MG who were anti-AchR and resistant to other therapies.
MG is rare in Prime’s commercial population. A 2018 study found 1,574 members with MG across 15 million commercially insured members.2 This new FDA-labeled, MG indication for eculizumab puts 75 to 85 percent of them within the new label indication. This could have a large impact on drug spend.2
Eculizumab cost projections
Without clinical programs in place to manage eculizumab, the PMPM impact could exceed $2.10. This is more than twice the expected PMPM of $0.94 with UM. This large difference is because eculizumab use could be used more broadly than for whom the manufacturer established it was effective. The UM criteria would ensure that the patients who receive eculizumab for MG are patients for whom the clinical trials have proven it will work.
Is this a trend in FDA labeling?
Eculizumab is not alone. Other drugs have received FDA labeling for indications broader than their supporting clinical trials.
Here are two more examples
1.Nusinersen (Spinraza®) was approved by the FDA on Dec. 23, 2016 for the treatment of spinal muscular atrophy (SMA) in pediatric and adult patients.3 The FDA approval included both pediatric and adult SMA patients. This was a broader population than expected. The studies submitted to the FDA did not include any patients over the age of 15 at study entry.3
Spinraza costs $750,000 in its first year. Subsequent year costs are $350,000.4 If 25 percent of people with spinal muscular atrophy take Spinraza, it could add $0.38 in projected new PMPM drug spend.5
2. Edaravone (Radicava®) was approved by the FDA on May 5, 2017 for the treatment of amyotrophic lateral sclerosis (ALS), also called Lou Gehrig’s disease. The FDA approval included all people with ALS. A closer look at the clinical studies suggests that only a subgroup of 7 percent of those with ALS would benefit.6 There are no reliable markers to select which patients with ALS might respond to Radicava. Prime’s UM and forecasts suggest a 40 percent uptake.7 Radicava’s wholesale acquisition cost (WAC) is $146,000 per year.8
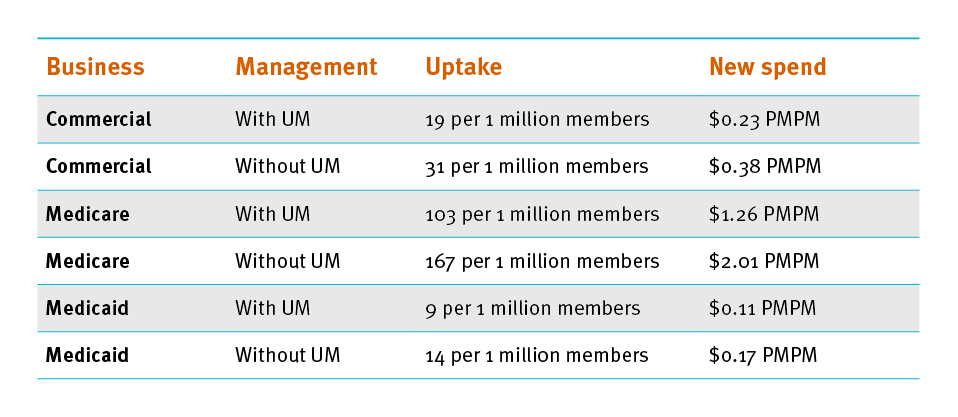
Radicava forecast by business line and drug management strategy
 (Forecasting and managing these drugs relies on integrated medical and pharmacy.)
(Forecasting and managing these drugs relies on integrated medical and pharmacy.)
All three of the drugs mentioned here process through the medical benefit rather than the pharmacy benefit. This means that UM will take the form of medical policy. From an adherence and care management perspective, it’s important to closely align the member cost share and the member experience across the medical and pharmacy benefit.
Prime has a pipeline management strategy focused on outcomes and analytics
The right pharmacy benefit manager (PBM) partner understands drug management strategy across both the medical and pharmacy benefits. Our drug management strategy has two key parts
- A UM policy in place by launch for products on either the medical or pharmacy benefit
- NetResults™ formulary management, our most aggressive formulary; (delivered $10 to $14 PMPM savings to clients last year)
We keep clients up to date with a communications strategy that includes:
- A Watch List drug trend forecast based on analytics of existing medical and pharmacy claims
- Drug trend forecast webinars that take a deep dive into forecasted prevalence, utilization and trend for emerging high cost therapies
- Drug Alerts that provide timely communications to key stakeholders, at the plan, with member-level impacts and trend forecasting by segment
- Weekly Prime Drug Insights Newsletters that provide summaries of drugs in the pipeline by category
- Drug Cost Calculator (available through our clinical program managers) that forecasts trend impact for high profile drug launches
This year, specialty reached 50 percent of total drug spend (medical and pharmacy). Our clients count on us for the information and tools to help them provide sustainable and quality health care.
References
- FDA Approves Soliris® (Eculizumab) for the Treatment of Patients with Generalized Myasthenia Gravis (gMG). Oct. 23, 2017. Haven CO. © 2018 Alexion Accessed at: http://news.alexionpharma.com/press-release/product-news/fda-approves-soliris-eculizumab-treatment-patients-generalized-myasthenia
- Howard JF Jr, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalized myasthenia gravis (REGAIN). Lancet Neurol 2017 Dec; 16 (12):976-986. https://www.ncbi.nlm.nih.gov/pubmed/29066163
- Starner CI, Vande Valle SE, Gunderson BW, Gleason PP. Exculizumab for Myasthenia Gravis: A Comprehensive Integrated Medical and Pharmacy Claims Analysis of Impact on Potential Uptake and Expenditures Among 15 million Commercially Insured Members. Poster Presentation at AMCP meeting; April 2018; Boston, MA. J Manag Care Spec Pharm 2018;24(4-a Suppl):S65. Accessed at: https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2018/document-amcpspring18-eculizumab.pdf
- FDA approves first drug for spinal muscular atrophy, Dec. 23, 2-16. U.S. Food and Drug Administration. Accessed at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm534611.htm
- “Costly Drug for Fatal Muscular Disease Wins F.D.A. Approval,” By Katie Thomas. Dec. 30, 2016. © 2018 The New York Times Company. Accessed at: https://www.nytimes.com/2016/12/30/business/spinraza-price.html
- Prime internal forecast on nusinersen.
- “Mitsubishi Tanabe’s ALS therapy, first new FDA-approved one in 20 years, now available in U.S.” by Emma Court. Published: Aug 8, 2017. Copyright © 2018 MarketWatch, Inc. Accessed at: https://www.marketwatch.com/story/mitsubishi-tanabes-als-therapy-first-new-fda-approved-one-in-20-years-now-available-in-us-2017-08-08-8914939
- Prime internal forecast on edaravone.
- “Edaravone: a new treatment for ALS on the horizon?” by Orla Hardiman, Leonard H van den Berg. Published: 15 May 2017. The Lancet.com website is operated by Elsevier Inc. Accessed at: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(17)30163-1/fulltext
Drug names are the property of their respective owners. NetResults is a trademark of Prime Therapeutics.
Related news
Perspectives
April 25, 2024
Drug Approvals Monthly Update: April 2024
This monthly update of United States (U.S.) Food and Drug Administration (FDA) approvals…
Perspectives
April 24, 2024
Prime/MRx resident wins AMCP Foundation Best Poster Award
Ai Quynh Nguyen, PharmD, was recently recognized for her research on opioid-prescribing patterns and outcomes
Perspectives
April 23, 2024
Expert Clinical Network Insights: April 2024
A look into our Expert Clinical Network (ECN) – part of Prime/MRx’s value-based approach to medical and pharmacy benefit management that offers access to more than 175 national and world-renowned key opinion leaders in multiple disease categories who provide expertise on challenging prior authorization case reviews, peer-to-peer discussions, drug policy development and formulary guidance