Perspectives
Small populations, big dollars, Part 1
Hemophilia: improving care and controlling costs
June 13, 2018Hemophilia. Some specialty conditions are rare, challenging to treat and difficult to live with. And the annual drug therapy price tag for members for these conditions can top $1 million. With stakes this high, specialty drug management becomes even more important.
Read about three of them here. Part 1: Hemophilia. Part 2: Myasthenia gravis. Part 3: Hereditary Angioedema.
Minding the details
With good care and treatment, people with hemophilia can live full and productive lives. We know that treatment for this condition is expensive. How can we make sure that treatment is sustainable for the health care system? Where is the line between cost-efficient and cost-inefficient care?
Blood basics: What it is and how to treat it
When a person has hemophilia, their blood doesn’t clot properly because it doesn’t have enough of the protein that causes clotting. Hemophilia is an inherited genetic disorder. There are two types of hemophilia, A and B. Hemophilia A is four times as common as hemophilia B. People with hemophilia A have inadequate clotting factor VIII, or FVIII. People with hemophilia B have inadequate clotting factor IX or FIX. Someone with hemophilia A or B will be diagnosed as mild, moderate or severe, based on how much FVIII or FIX they have, respectively.¹
Most often, the factor protein is replaced through infusions of clotting factor concentrates. Infusions can help stop excessive bleeding, and given regularly, can help prevent severe bleeding from happening.
Treatment progress has lengthened life expectancy from 20 years to near normal
In the 1930s, some people with hemophilia were treated with diluted snake venom. In the late ‘50s, fresh frozen plasma was transfused in the hospital. In the ‘70s, freeze-dried powdered concentrates containing factor could be stored to be self-infused at home. By the 1990s, prophylaxis (preventive) factor treatment two or three times a week was helping many people with hemophilia lead regular lives.²
Today, the factor product category includes more than two dozen agents with more in the pipeline. And gene therapy for a cure is in clinical trials. But we still need to afford today’s treatments.
An analysis Prime conducted in 2015 showed that annual drug costs for members with using hemophilia factor products ranged from about $1,000 to just over $3 million.³
It’s rare, and there are complications
You may not know anyone with hemophilia. There aren’t very many. Only 20,000 in the whole United States. Across Prime’s 15 million-member commercially insured population, a recent analysis only found about 660 members with the two types of hemophilia, A and B.4
Over time, about 15 to 30 percent of people with hemophilia develop an antibody (inhibitor) that stops clotting factors from working properly.5 That makes it harder to treat excessive bleeding. These people need special treatment for their hemophilia until their bodies stop making the inhibitors. They might use high-dose clotting-factor concentrates, bypassing agents or immune-tolerance induction therapy. That makes treatment costs go up.
Factor doesn’t last long in the system
All drugs have a half-life. The half-life of a drug is the time it takes for the concentration of the drug in the body to reduce to half its peak amount.
Until recently, all hemophilia A factor VIII products had a standard half-life (SHL) in the system of a person with hemophilia. Half of the clotting factor which was infused was removed by the body every 12 to 24 hours. This means that within 2 or 3 days almost none is left. Recently, a few extended half-life (EHL) factor products were introduced. They were much more expensive, but the idea was that someone with hemophilia would need less factor because they were taking infusions less frequently.
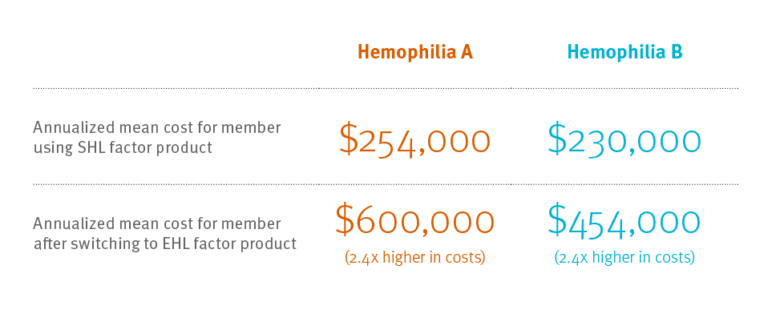
A 2018 Prime analysis looked at costs and use of changing from SHL to EHL
Expected results: Prime’s study found that when members with hemophilia B, factor IX deficiency, changed from standard half-life (SHL) to extended half-life (EHL) factor products, the members used one-third less factor product.3This can mean the difference between someone having factor infusion every other day and having factor infusion once a week. That’s a major improvement. This was what we expected. (Even with using less factor, because EHL factor costs more, costs doubled.)
Unexpected results: Members with hemophilia A that switched from SHL to EHL factor VIII products used more factor product, not less. And because EHL factor costs so much more, these members incurred costs that were 2.4 times as much as before.5 More factor and 2.4 times more cost? Where’s the clinical value in that?
Four of five Prime members with hemophilia A still use SHL factor VIII product. Many of these members may want to move to EHL factor product. They may expect to take less factor product, but it’s not likely, based on this research. And their costs will likely more than double.
Prime forecast
Prime’s recommendations
Prime recommends utilization management (UM) and/or medical policy criteria for EHL factor product.
Prime has engaged the National Hemophilia Foundation with these findings.
Prime also recommends care management for hemophilia. Close monitoring with care management can support treatment plans and monitor in-home supplies.
Prime recommends pharmacokinetic testing when considering an EHL.6
In hemophilia, UM and care management strategies can help improve the quality of care and potentially save tens, even hundreds of thousands of dollars per member.
References
- Fast Facts. National Hemophilia Foundation. (n.d.) © 2018 National Hemophilia Foundation. Accessed April 1, 2018 at https://www.hemophilia.org/About-Us/Fast-Facts
- History of Bleeding Disorders. National Hemophilia Foundation. © 2018 National Hemophilia Foundation. Accessed at: https://www.hemophilia.org/Bleeding-Disorders/History-of-Bleeding-Disorders
- Bowen K L, Gleason PP. 2012 Prevalence and Cost of Coagulation Factor Treatment for Hemophilia and von Willebrand’s Disease Among 10 Million Commercially Insured Members. J Manag Care Pharm 2013;19(8):655.
- Bowen K, Borchard M, Gleason PP. Incremental Cost of Switching to Extended Half-life (EHL) Coagulation Factor Products to Treat Hemophilia Among 15 Million Commercially Insured Members. April 2018. AMCP Boston, MA. Poster Presentation. https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2018/document-amcpspring18-hemophilia.pdf
- The challenges of inhibitors. National Hemophilia Foundation. (n.d.) National Hemophilia Foundation. © 2018 National Hemophilia Foundation. Accessed at: https://www.hemophilia.org/Bleeding-Disorders/Inhibitors-Other-Complications/Inhibitors-for-Providers/The-Challenges-of-Inhibitors
- Iorio A, Blanchette V, Blatny J, et al. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of ISTH. J Thrombosis Haemostatis 2017;15:2461-2465
Related news
Perspectives
April 17, 2024
AMCP 2024: Behind the research with YuQian Liu
Ahead of her session with Andy Killpack, Liu — senior director of specialty clinical solutions at Prime/MRx — shares current care management strategies for cell and gene therapy and the future of this exciting frontier
Perspectives
April 16, 2024
AMCP 2024: Behind the research with Jacob LaRue and Timothy O’Shea
Ahead of their session, Jacob and Timothy share how Prime/MRx is working alongside providers like Horizon Blue Cross Blue Shield of New Jersey to manage drug waste and rein in spend for specialty drugs without therapeutic impact to patients
Perspectives
April 15, 2024
Oncology Insights: Cancer treatment is personal
Precision medicine, or personalized medicine, uses genes or proteins to diagnose or treat disease. This medical care design has significantly impacted oncology and grew out of a need to improve and individualize patient treatments
 PharmD, FCCP, FAMCP, BCPS | Assistant Vice President, Health Outcomes
PharmD, FCCP, FAMCP, BCPS | Assistant Vice President, Health Outcomes