Perspectives
Rheumatoid arthritis: Finding the right treatment can take years, Part 2
Patients work with their doctors; a treatment plan may need to change
December 27, 2017There is still no cure for rheumatoid arthritis (RA), but there are better and more targeted treatment options. These treatment options can allow members with RA to slow and sometimes even stop the progression of the disease. The right medicine regimen can help members with RA reduce pain, remain active and live fuller lives.
Diagnosing RA can be a challenge
The symptoms of rheumatoid arthritis can come on suddenly, most commonly as swelling and pain in finger or toe joint. However, because symptoms vary and often resemble other conditions, it can take months or even years to get a correct diagnosis. Studies show that the sooner treatment begins after symptoms develop, the more likely it is for joint damage to slow or even stop.1
The drugs that treat RA are called DMARDS: disease modifying antirheumatic drugs. They divide into two main categories – conventional synthetic DMARDs (csDMARDs) and newer, biologic or targeted synthetic DMARDs (b/tsDMARDs).
csDMARDs
- Methotrexate
- Hydroxychloroquine
- Leflunomide
- Sulfasalazine
b/tsDMARDs entered the marketplace beginning early in the 2000s.1
b/tsDMARDs
b/ts TNF inhibitors
- Humira (adalimumab
- Enbrel (etanercept)
- Cimzia (certolizumab pegol)
- Inflectra (infliximab-dyyb)
- Renflexis (infliximab-abda)
- Remicade (infliximab)
- Simponi/Simponi AIRA (golimumab)
b/ts non TNF inhibitors
- Actemra (tocilizumab)
- Orencia (abatecept)
- Rituxan (rituximab)
- Kineret (anakinra)
- Kevzara (sarilumubab
(non-biologic targeted synthetic agents)
- Xeljanz/Xeljanz XR (tofacitinib)
- Other agents in the pipeline
Does it matter to the PBM what drugs a member takes?
Prime wants members to get the medicine they need to feel better and live well. That means following best clinical guidelines. And that also means keeping health insurance affordable.
Treatment guidelines for RA recommend csDMARD monotherapy as first line therapy. Methotrexate is preferred. Treatment guidelines further suggest the use of b/tsDMARDs only after application of triple therapy of csDMARDs (defined as using three csDMARDs in combination).
Recent clinical studies compared the cost and effectiveness of treatment with triple therapy vs. moving directly to a b/tsDMARD. csDMARDs cost a few dollars per day compared to b/tsDMARDs which cost $100 per day. Using triple therapy before starting b/tsDMARDs can provide effective care and greatly reduces RA costs.2-6
Multiple studies have found that protocols that use a treat to target strategy and csDMARDs are not inferior to treatment that turns immediately to the newer, vastly more expensive b/tsDMARDs. And csDMARD remission can persist longer than those achieved with b/tsDMARDs.7-9
Maximizing use of csDMARDs will save money
Total medical plus pharmacy claims of for RA members treated with b/tsDMARDs costs are 2.5x more than for RA with csDMARDs.10 Optimizing csDMARD therapy can hold down the cost of care.
That makes a big difference.
Room for improvement
Prime’s research demonstrates RA treatment guidelines aren’t being followed often enough.11
Cost comparison among members on csDMARDS, members on b/ts DMARDS and matched members not on specialty drugs
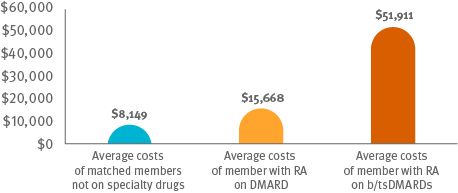
Following guidelines: It appears that only 4 percent of members received treatment that followed guidelines, using triple therapy of csDMARDs before progressing to b/tsDMARDs.
Moving from csDMARDs to b/tsDMARDs too quickly: More than one third of members initiating b/tsDMARD who did have an earlier csDMARD claim were only on the csDMARD for just 12 to 24 months. Guidelines suggest it can take at least 12 weeks of treatment to evaluate a regimen. These members and their doctors may only have had time to make one or two adjustments in their regimen.
Skipping csDMARDs and going straight to b/tsDMARDs: Another 10 percent of members that initiated b/tsDMARDs had no claim for a csDMARD at all in the previous 12 months.
No DMARDs at all: Nearly half of members newly diagnosed with RA did not have any claim for any DMARD within 12 months of diagnosis. Almost 40 percent had not DMARD claim within 24 months.
To optimize csDMARD use, utilization management tools like step therapy can be used. This can encourage the trial of more than one csDMARD before a biologic therapy is initiated.
Through the analysis of pharmacy and medical data, Prime’s GuidedHealth® program provides actionable clinical intelligence to doctors, members and plans. These insights can result in improved care, safer medicine use, better outcomes and lower overall cost of care.
The last article in this series looks at specific ways a plan sponsor and PBM can help improve the RA standard of care.
References
- Peper SM, Lew R, Mikuls T, et al. Rheumatoid arthritis treatment after methotrexate: triple therapy is more durable than etanercept. Arthritis Care Res 2017; article accepted.More cost effective
- Jalal H, O’Dell JR, Bridges SL, et al. Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthritis Care Res 2016; 68(12)):1751-1757.
- Bansback N, Phibbs CS, Sun H, et al. Triple therapy versus biologic therapy for active rheumatoid arthritis: A cost-effectiveness analysis. Ann Int Med 2017;167:8-16.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. NEJM 2014;371:396-397.
- Hazlewood GS, Barnabe C, Tomlinson G, et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 2016;353:I 777.
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004; 364:263-269.
- Rannio T, Asikainen J, Hannonen P, et al. Three out of four disease-modifying anti-rheumatic drug-naïve rheumatoid arthritis patients meet 28-joint disease activity score remission at 12 months: results from the FIN-ERA cohort. J Rheumatol 2017;1-17.
- Peper SM, Lew R, Mikuls T, et al. Rheumatoid arthritis treatment after methotrexate: triple therapy is more durable than etanercept. Arthritis Care Res 2017; article accepted.
- Bowen KL, Gleason PP. Rheumatoid arthritis 2016 prevalence, drug treatment, and total medical and pharmacy claims expense in a 15 million member commercially insured population. Poster presentation AMCP October 2017. J Manag Care Pharm 2017:23(10-a):S74. Accessed at https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2017/document-amcpposter2017-ra.pdf
- Bowen KL, Gleason PP. Incidence Rate of Biologic/Targeted Synthetic (b/ts) Disease Modifying Antirheumatic Drugs (DMARDs) for Rheumatoid Arthritis (RA), Preceding Therapy and Time to Discontinuation in a Commercially Insured Population. Poster presentation AMCP October 2017. AMCP 2017. J Manag Care Pharm 2017:23(10-a):S73. Accessed at: https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2017/document-amcpposter2017-radmards.pdf
GuidedHealth is a registered trademark of Prime Therapeutics LLC. Drug names are the property of their respective owners.
Related news
Perspectives
April 17, 2024
AMCP 2024: Behind the research with YuQian Liu
Ahead of her session with Andy Killpack, Liu — senior director of specialty clinical solutions at Prime/MRx — shares current care management strategies for cell and gene therapy and the future of this exciting frontier
Perspectives
April 16, 2024
AMCP 2024: Behind the research with Jacob LaRue and Timothy O’Shea
Ahead of their session, Jacob and Timothy share how Prime/MRx is working alongside providers like Horizon Blue Cross Blue Shield of New Jersey to manage drug waste and rein in spend for specialty drugs without therapeutic impact to patients
Perspectives
April 15, 2024
Oncology Insights: Cancer treatment is personal
Precision medicine, or personalized medicine, uses genes or proteins to diagnose or treat disease. This medical care design has significantly impacted oncology and grew out of a need to improve and individualize patient treatments