Perspectives
Ground yourself in the basics about biosimilar drugs, Part 1
The place to go when all you need are the basics
March 14, 2023- Part 1: What are biosimilar drugs?
- Part 2: Biosimilar naming conventions: Top 10 acronyms to know
- Part 3: Getting biosimilars to market–what works and what doesn’t.
- Part 4: Biobetters vs biosimilars: What’s the difference?
It’s okay not to know (or remember) all of the answers all of the time. Let’s get started.
What is a biologic product?
Biologic products are used to diagnose, prevent, treat and cure diseases and medical conditions. Most are used to treat a wide range of specialty diseases and conditions, including many cancers and orphan diseases. They may be produced from living organisms or contain components of living organisms.
There are many types of biological products approved for use in the United States, including:
- Therapeutic proteins (such as filgrastim)
- Monoclonal antibodies (such as adalimumab) and
- Vaccines (such as those for influenza and tetanus)
What are biosimilar drugs?
A biosimilar product is manufactured to work like its reference biologic. (The original FDA-approved biologic is often called a “reference product.”) A biosimilar has no clinically meaningful differences from the reference product. In other words, it is FDA-approved to be just as safe and as effective as the original biologic.1
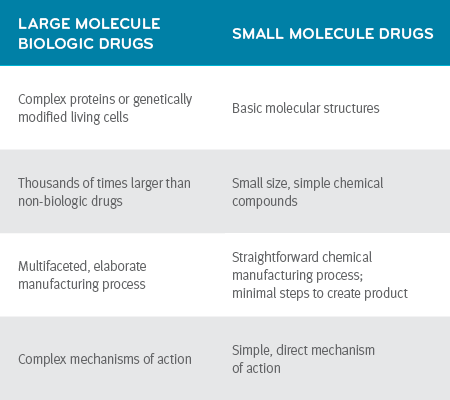
What’s the difference: biosimilars vs generic?
 When it comes to biosimilars and generics, it’s important to remember that they’re both alternative versions of brand-name drugs. They can be manufactured after the brand-name drugs’ patents have expired.
When it comes to biosimilars and generics, it’s important to remember that they’re both alternative versions of brand-name drugs. They can be manufactured after the brand-name drugs’ patents have expired.
A generic has the same active ingredients as its brand-name counterpart. An FDA approval of a generic means the generic manufacturer has shown, with clinical studies, that the generic performs the same way in the human body as the brand-name drug. The generic drug is equivalent to the brand-name drug in dosage, form, safety, strength, route of administration, quality and performance.2
A biosimilar won’t be identical to its reference product. It will be similar. But similar still gets the job done. Prescribers and patients should have no concerns about using biosimilar products vs their reference products.
Biosimilars have a separate approval process and are required to provide the FDA with different documentation vs generic drugs.
Once approved by the FDA, biosimilars and their biologic reference products have been shown to have no clinically meaningful differences in dosage, form, safety, strength, route of administration, purity or potency.3
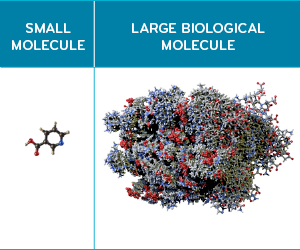
 Biologics can be thousands of times larger than small molecule drugs. For example, Humira (adalimumab), the nation’s top drug by revenue, contains 20,067 atoms.
Biologics can be thousands of times larger than small molecule drugs. For example, Humira (adalimumab), the nation’s top drug by revenue, contains 20,067 atoms.
Small molecule drugs have basic molecular structures. Their chemical make-up is compact. For example, Lipitor (and its generic equivalent, atorvastatin) has 76 atoms.
In January 2017, the FDA published a guidance document that recommended biologic and biosimilar manufacturers add a four-letter suffix to the proper names of products. The four letters are a meaningless combination of lower-case letters that reflect a consistent naming convention.5 It’s not an indicator of product quality.
How are biosimilar products named? What’s with the weird four-letter suffixes?
Adding the suffixes to the names of biosimilar products was intended to:
- Help differentiate products in a drug class with several biosimilars
- Support patient safety by making it easier to monitor manufacturer-specific biologic products and to track any related adverse events
- Help distinguish between different versions of medicines and biosimilars in the same drug class
The FDA did not make this guidance retroactive. Because the biologic products approved before 2017 don’t have the suffix, it may appear like the guidance only applies to biosimilar products. But from 2017 forward, this practice has been applied to all biologic and biosimilar products.4
Who makes biosimilars? Why do we have them?
The same drug manufacturers that make biologic products make biosimilars. We have biosimilars for the same reason we have generic drugs. They are part of an effort to lower drug costs and increase therapeutic options. According to data from the IQVIA Institute, biologic drugs have accounted for nearly all (93 percent) of the net drug spending growth since 2014. This makes savings from biosimilars critical to managing the rising costs of biologics. How many biosimilars are on the market in the U.S. today?
As of February 2023, 40 biosimilars had been approved by the U.S. FDA. Only 27 have been launched. Eighteen biosimilars are in the pipeline seeking FDA approval.
The U.S. biosimilar market is starting to gain momentum. The potential savings from biosimilars is estimated between $24 and $150 billion from 2017 to 2026.5 That’s a huge range.
The bottom line? Since biosimilars can be used with no impact to quality of care, the decision to lower health care costs by using biosimilars will depend on effort and commitment from those of us in the industry.
What’s next?
Learn more about how biosimilars help lower drug costs while maintaining patient care by visiting our Biosimilars page.
Want to talk about how adding them to your plan could benefit both you and your members? Contact us directly.
References
- Biosimilar and Interchangeable Products. 10-17-17. US FDA. Fda.gov. Accessed at: https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products
- Overview and basics. 9/13/17. US FDA. FDA.gov. Accessed at: https://www.fda.gov/drugs/generic-drugs/overview-basics
- Abbreviated new drug application (ANDA). FDA. Updated May 22, 2019. Accessed at: https://www.fda.gov/drugs/types-applications/abbreviated-new-drug-application-anda
- Nonproprietary Naming of Biological Products: Guidance for Industry. Food and Drug Administration. January 2017. Labeling. Accessed at: https://www.fda.gov/media/93218/download
- Biosimilar Cost Savings in the United States: Initial Experience and Future Potential. Mulcahy AW, Hlavka JP, Case SR. Rand Health Q. 2018 Mar 30;7(4):3. eCollection 2018 Mar. Accessed at: https://www.ncbi.nlm.nih.gov/pubmed/30083415
Related news
Perspectives
April 23, 2024
Expert Clinical Network Insights: April 2024
A look into our Expert Clinical Network (ECN) – part of Prime/MRx’s value-based approach to medical and pharmacy benefit management that offers access to more than 175 national and world-renowned key opinion leaders in multiple disease categories who provide expertise on challenging prior authorization case reviews, peer-to-peer discussions, drug policy development and formulary guidance
Perspectives
April 23, 2024
April Fraud Focus – How to prioritize drug safety at home
While ensuring drug safety is often considered the responsibility of pharmaceutical industry players,…
Perspectives
April 19, 2024
AMCP 2024: Behind the Research with Prerak Parikh
Parikh, director of medical pharmacy strategy at Prime/MRx, shares the latest on interchangeable biosimilars