Perspectives
Gene therapy opens new worlds in health care
KymriahTM launches great expectations
November 2, 2017What’s ahead of us? There’s a lot we don’t know. Let’s start with what we do know.
Not just another drug
The FDA approved Kymriah on August 30, 2017. It’s a genetically modified T-cell immunotherapy for the treatment of patients up to 25 years of age with second or later relapsed and refractory (r/r) B-cell acute lymphoblastic leukemia (ALL). In one clinical trial, a single treatment with Kymriah, priced at $475,000, resulted in remission in nearly all patients.1 The patient’s own immune cells are genetically modified to target and kill leukemia cells. Kymriah is sparking a sea change in the economics of health care. We don’t yet know where this will lead. But we cannot pretend it will be business as usual.
Kymriah treats a narrow sliver of patients with leukemia
ALL is the most common childhood cancer. It occurs when bone marrow cells develop errors in their DNA. Fewer than 200,000 children in the United States will be diagnosed with ALL.2 Symptoms may include enlarged lymph nodes, bruising, fever, bone pain, bleeding from the gums and frequent infections. Treatments include chemotherapy or targeted drugs; these treatments successfully result in remission in 85 percent of children with ALL.3
A few hundred children and young adults in the United States are diagnosed with resistant or relapsing ALL each year.4 They have undergone years of difficult treatments for a condition that is itself, draining and debilitating. These patients have limited treatment options; at this point, the treatment-resistant disease is usually fatal.4 Until now.
It’s not just remission, it may be a cure. The FDA approved Kymriah based on a trial of 63 severely ill children and young adults. The trial showed a remission rate of 83 percent within three months.7
The gene therapy treatment process itself is unique
Kymriah’s chimeric antigen receptor T-cell (CAR-T) therapy alters the patient’s own T-cells to fight the cancer. Although the treatment is a single infusion, the entire process takes several weeks.5 It includes:
- Leukapheresis: The patient is admitted to one of 32 medical centers across the country approved to conduct the procedure. A health care team removes whole blood from the patient. T-cells are separated, frozen and shipped to a Novartis lab.
- Production and multiplication of CAR T-cells: At the lab, the T-cells are genetically engineered to produce chimeric antigen receptors (CAR) on the T-cell surface: they become cancer-killing cells. These CAR T-cells are grown until they number in the billions. This takes about three weeks.
- Lymphodepleting/conditioning chemotherapy: While the CAR T-cells are growing in the laboratory, the patient is given chemotherapy. This helps to create a favorable environment, so when the CAR T-cells are infused, they can continue to grow in number.
- Reinfusion of Kymriah cells: The Kymriah cells are reinfused into the patient. The CAR T-cells target the patient’s cancer cells and begin to destroy the cancer cells.
- Monitor patient for adverse events: Patients may need to be hospitalized due to side effects associated with Kymriah. These may include cytokine release syndrome (CRS), a known and serious complication of CAR T-cell therapy. (CRS can indicate a better response to therapy.5)
Dr. Carl June, a leader in developing the treatment at the University of Pennsylvania, recalls the early trials in 2010. “I have to keep pinching myself to see that this happened…I think the cancer world is forever changed.”4
Potential impact on drug spend
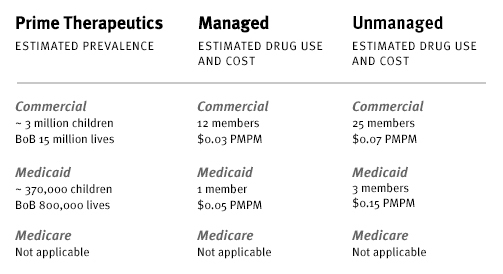
Prime has followed Kymriah on its drug watch list for several months. Prime’s proprietary drug cost calculator estimates the number of individuals with a diagnosis who would potentially need to be treated with this high-cost therapy. The calculator uses estimates of pediatric and young adults with (r/r) B-cell ALL. It also uses demographic information from Prime’s book of business.
The following represents estimates of drug use and cost for Kymriah, managed and unmanaged. Managed suggests that 50 percent of those diagnosed would receive therapy. Unmanaged suggests that 100 percent of those diagnosed would receive therapy. Kymriah, and costs associated with the treatment process, are expected to impact the medical benefit.
Kymriah projected utilization and cost
Novartis has said that if a patient does not respond to treatment, the company will not charge for the drug.4 But many of the additional therapy costs associated with the administration of Kymriah are not included in the price of $475,000 (e.g., leukapherisis, conditioning chemotherapy, hospitalizations, adverse event treatment for CRS, etc). Experts say those costs may total $1 million or more.9
Patient assistance programs and travel assistance may be available to eligible underinsured patients, according to a Novartis spokesperson.8
The health care marketplace is getting more complicated, not less. Prime will continue to work with our Blue Plan owners toward productive and positive answers to the challenges ahead.
References
- Statnews. Novartis wins a landmark FDA approval with first CAR-T cancer drug. Accessed in August 2017 at https://www.statnews.com/2017/08/30/novartis-car-t-cancer-approved
- “What is Childhood Leukemia?” American Cancer Society. Accessed at: https://www.cancer.org/cancer/leukemia-in-children/about/what-is-childhood-leukemia.html
- “Survival rates for childhood leukemias.” American Cancer Society. Accessed at: https://www.cancer.org/cancer/leukemia-in-children/detection-diagnosis-staging/survival-rates.html
- “F.D.A. Approves First Gene-Altering Leukemia Treatment, Costing $475,000,” by Denise Grady. Aug 30, 2017. New York Times. Accessed at: https://www.nytimes.com/2017/08/30/health/gene-therapy-cancer.html?action=click&contentCollection=Health&module=RelatedCoverage®ion=EndOfArticle&pgtype=article
- LLS.org Chimeric antigen receptor (CAR) T-cell therapy. Accessed in June 2017 at: https://www.lls.org/treatment/types-of-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy
- “New Gene-Therapy Treatments Will Carry Whopping Price Tags,” By Gina Kolatasept, Sept. 11, 2017. New York Times. Accessed at: https://www.nytimes.com/2017/09/11/health/cost-gene-therapy-drugs.html
- Kymriah (tisagenlecleucel) suspension for intravenous infusion. Prescribing information. Novartis Pharmaceuticals. Accessed in October 2017 at: https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM573941.pdf
- “Kymriah, the first gene therapy, arrives with a $475,000 price tag.” By Ginger Skinner, September 3, 2017. Consumer Reports. Accessed at: https://www.consumerreports.org/drug-prices/kymriah-first-gene-therapy-costs-475000-dollars-childhood-cancer/
- “Cascade of costs could push new gene therapy above $1 million per patient,” By Liz Szabo. Oct. 17, 2017. Kaiser Health News. 2017 Kaiser Family Foundation. Accessed at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
Drug names are the property of their respective owners.
Related news
Perspectives
April 19, 2024
AMCP 2024: Behind the Research with Prerak Parikh
Parikh, director of medical pharmacy strategy at Prime/MRx, shares the latest on interchangeable biosimilars
Perspectives
April 19, 2024
LISTEN NOW: Live at AMCP Annual 2024 – Digging into managed care pharmacy insights | Pharmacy Friends podcast
In this episode, Prime/MRx clinicians — along with special guests — discuss the hottest topics covered at the Academy of Managed Care Pharmacy (AMCP)'s 2024 Annual Meeting in New Orleans
Perspectives
April 17, 2024
AMCP 2024: Behind the research with YuQian Liu
Ahead of her session with Andy Killpack, Liu — senior director of specialty clinical solutions at Prime/MRx — shares current care management strategies for cell and gene therapy and the future of this exciting frontier