Perspectives
Entresto®: Take down the UM gate
Taking this for heart failure was associated with lower cost of care.
December 28, 2020Heart failure affects more than 6.5 million people in the U.S.¹ It is the leading cause of hospitalization in people older than 65. It causes more deaths than cancer (or COVID).³,4 The number of people diagnosed with heart failure is projected to rise 46 percent by 2030, according to the American Heart Association.²
Heart failure describes when the heart cannot pump enough blood and oxygen to support other organs in the body. It’s chronic and progressive. Symptoms include shortness of breath, fatigue and weakness, rapid or irregular heartbeat, a reduced ability to exercise, fluid retention and a persistent cough or wheezing. Patients often develop heart failure after their hearts have been weakened or damaged by other cardiovascular conditions.
Leading Causes of Death and Number of Deaths for the Leading Causes of Death 3,4
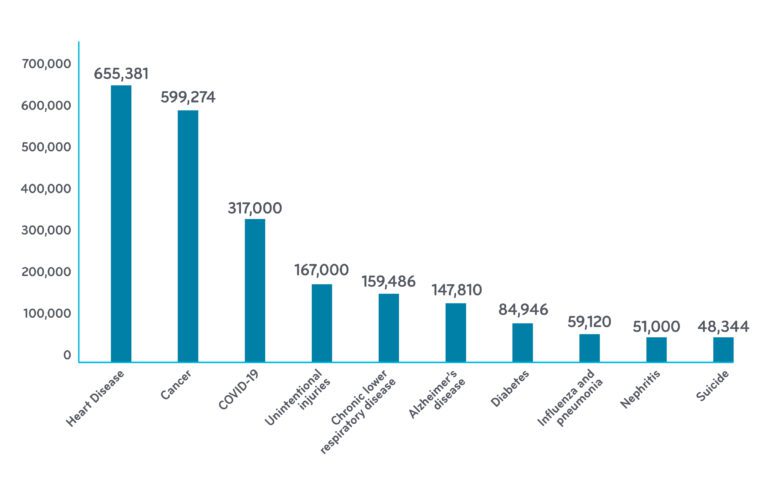
Data for the U.S. from 2018 (except COVID: Jan-Dec 18 2020)
Studies show new drug’s benefit to heart failure patients
Entresto® (sacubitril/valsartan, or sac-val) was FDA-approved in 2015 to treat heart failure with reduced ejection fraction (HFrEF). Reduced ejection fraction means the muscle of the heart’s left ventricle is not pumping strong enough to get needed blood into circulation. (HFrEF is found in 40 to 60 percent of patients with heart failure.5)
In 2016, sac-val was added to treatment guidelines for the treatment of heart failure with HFrEF. One author of the new guidelines called it the “dawning of a new era for the treatment of heart failure.”6
Sac-val’s ability to reduce mortality has been documented, but its uptake has been slow for a number of reasons:7
- Most heart disease drug treatment protocols are well established and consist primarily of generic drugs.
- Use of sac-val generally requires a doctor to switch a patient from a traditional therapy like an ACE inhibitor. Many doctors are hesitant to switch to another drug, if the current drug is working well.5 It is work for the doctor to convert to sac-val because sac-val can cause a blood pressure drop when starting therapy, therefore close doctor monitoring is required.
- Cardiologists are not used to filling out prior authorization (PA) forms; they may avoid prescribing drugs with PAs. (Other specialties that see new drugs every year – like rheumatologists, neurologists, or oncologists – fill out a lot more PAs.)
Studies like Prime’s provide valuable real-world data
Beyond the manufacturer’s own trials, little was known of sac-val’s real-world impact on total cost of care (TCC). Third party research, like the kind Prime does, often provides new and different data, like use of medical services over time, variability of adherence rates and complete cost of care.
Prime uses integrated medical and pharmacy claims data for its studies
Prime’s study identified 1,036 members newly initiating sac-val that had enrollment one year prior and one year post their sac-val start date. The mean age of the population was 54 years, and 73 percent of members were male.8
The study group narrowed to 658 members when we focused in on those who had good adherence to sac-val during the study period. We measured good adherence as percentage days covered (PDC) ? 80 percent.
The study divided the total cost of care into pharmacy costs and medical costs, hospitalizations, ER, office visits, and all other. This real-world analysis includes actual use of health care services and insurance paid costs that can’t be found in a clinical trial, and therefore more useful to a health plan.
The results of the analyses surprised even me. For an adherent member taking sac-val, after just one year:
- Total cost of care decreased 22 percent, from $46,000 to $36,000
- Medical costs decreased a startling 38 percent, from $42,000 to $26,000.
Pre and Post Total Mean Costs for Sac-Val HFrEF Adherent Utilizers
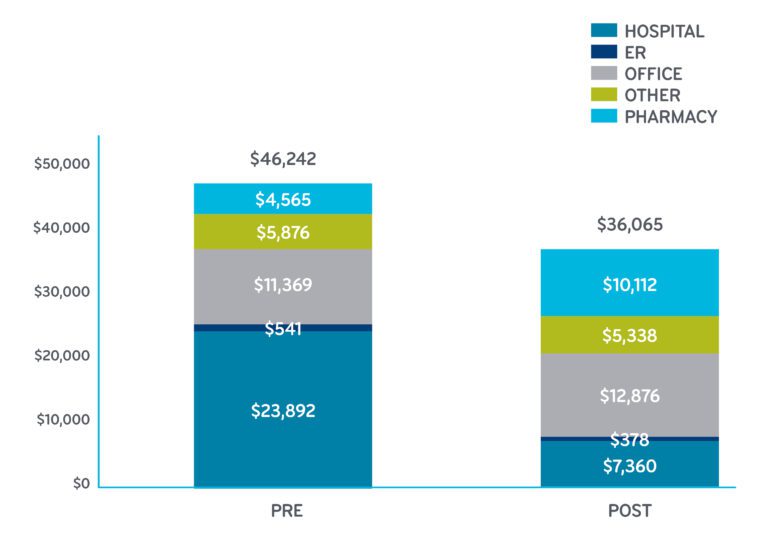 Health care consumer price index (CPI) adjusted costs. Sac-val costs after adjustment for formulary access rebate and administrative fees; all other pharmacy costs unadjusted.
Health care consumer price index (CPI) adjusted costs. Sac-val costs after adjustment for formulary access rebate and administrative fees; all other pharmacy costs unadjusted.
Pharmacy costs go up, but medical costs come down
Pharmacy: You see the increase in the pharmacy cost. Sac-val is only available in the brand-name drug Entresto, which costs about $4,000 per member per year (PMPY). This includes the real-world patient use and all discounts. The sac-val actual cost is included in the increase of pharmacy costs from $4,565 to $10,112. Sac-val accounted for 72 percent of that increase. (As you look at these charts, keep in mind that heart failure-related pharmacy costs include a wide range of medications, including sac-val, ACE/ARB, beta blockers, digoxin, aldosterone antagonists, hydralazine and isosorbide dinitrates, ivabradine, and loop diuretics.)
Medical: For patients adherent to sac-val, the big impact is associated with the 69 percent decrease in hospital costs and 44 percent decrease in emergency room costs. Costs for office visits are associated with a small 5 percent increase.
Change in Use of Medical Services By Percentage
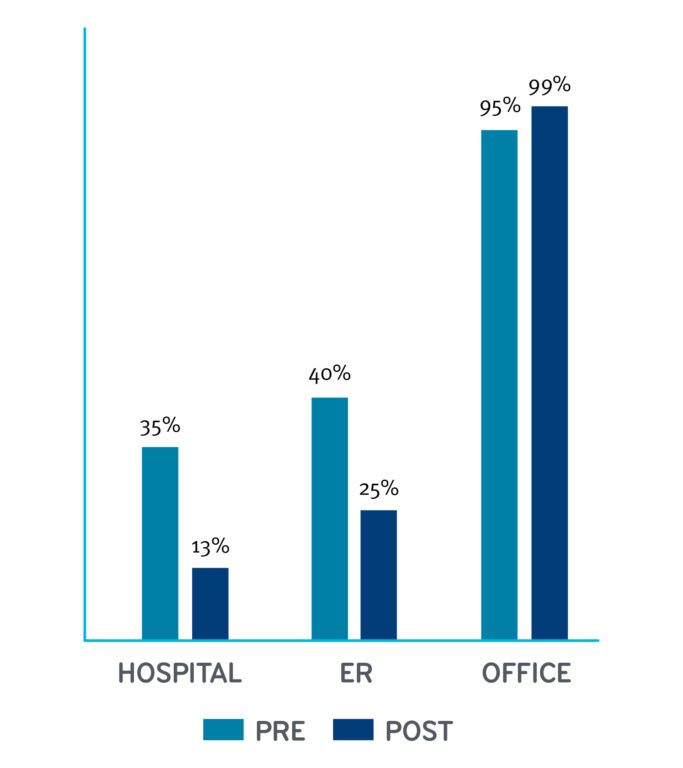
It’s worth noting the use of services as well as the cost. Thirty five percent of patients had a hospital stay during the pre-index period; after starting sac-val, the percentage of patients that had a hospital stay dropped dramatically to 13 percent. The number of patients using emergency room services had a similar dramatic drop in use.
What about the patients who weren’t adherent?
We started the study with 1,036 patients, new to sac-val. We had to trim the group to 658 to focus on the 658 patients with 80 percent adherence. What would happen if we included the 378 weren’t as adherent as the rest of the cohort? We did an analysis of the entire group – all 1,036, This was important to do to represent real world data: in the real world, not everybody stays adherent.
The bigger group also showed benefit from taking sac-val, just not as much
When we included all 1,036 patients, regardless of adherence, the group as a whole still showed a small total cost of care decrease (2 percent).
Here’s how the results were different: The pharmacy cost increases were the same. The medical costs dropped, but not as much. The medical costs didn’t drop enough to show the same big savings of the smaller, more adherent group. But that still makes sac-val a bargain from Prime’s perspective. Easy on the budget, saves lives.
This makes an open formulary offering of sac-val a neutral decision from a cost perspective, and a good decision from a health outcomes perspective.
This makes it the right decision to call for unrestricted use of this drug for appropriate patients. We were able to remove the prior authorization on this drug.
PAs have a role to ensure safe, cost-effective therapy selection
Prior authorizations in utilization management provide important safeguards for pharmacy benefit management for many reasons. One role of a PA is to ensure the safest medication is selected. Because some doctors may want to use Entresto to treat high blood pressure or for less severe heart failure, for which Entresto does not have an FDA-approved indication, some insurers have a sac-val PA in place as a safety check due to the low blood pressure risk when initiating therapy. But the heart failure guidelines are clear. Our own real-world data shows sac-val benefit. There are concerns that a PA could be a barrier to sac-val access, and we know a lot of doctors’ office systems are still not set up to manage PAs efficiently.
A survey from the American Medical Association from a couple of years ago says that PAs interfere with continuity of care.10 So, eliminating a PA can give the member faster access to sac-val, which can save lives and health care costs.
 Yes, Entresto is more expensive than other treatments – but only if you are just looking at the pharmacy costs. Prime looks at the integrated total cost of care. And the total cost of care offset is significant. We highly recommend to our health plans and self-insured employers to remove any Entresto PA. We encourage the appropriate use of this life-saving, cost-effective medication for HFrEF.
Yes, Entresto is more expensive than other treatments – but only if you are just looking at the pharmacy costs. Prime looks at the integrated total cost of care. And the total cost of care offset is significant. We highly recommend to our health plans and self-insured employers to remove any Entresto PA. We encourage the appropriate use of this life-saving, cost-effective medication for HFrEF.
We know health care is hard. We work hard to provide the data to make decisions easier. Here’s an easy decision. If a patient has HFrEF, using sac-val is associated with lower cost of medical services and a neutral impact on total cost of care. No PA needed. It improves patient outcomes without touching the budget. Sounds like a good prescription to me.
References
- Heart failure projected to increase dramatically, according to new statistics, By American Heart Association News, January 25, 2017. ©2020 American Heart Association, Inc. All rights reserved. Accessed on November 13, 2020. https://www.heart.org/en/news/2018/05/01/heart-failure-projected-to-increase-dramatically-according-to-new-statistics#:~:text=The percent20number percent20of percent20people percent20diagnosed,in percent20new percent20window) percent20published percent20Wednesday.
- Facts About Heart Failure in the United States. U.S. Department of Health & Human Services. USA.gov. Accessed November 13, 2020. https://www.cdc.gov/heartdisease/heart_failure.htm#:~:text=Facts percent20About percent20Heart percent20Failure percent20in,estimated percent20 percent2430.7 percent20billion percent20in percent202012
- Number of deaths for leading causes of death in the United States, 2018. U.S. Department of Health & Human Services, USA.gov. Accessed on November 16, 2020: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- 246,000 COVID deaths in US. as of November 15, 2020. New York Times. Accessed at: https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html
- Heart Failure, A presentation by Ryan J. Tedford, MD, FACC, FAHA, FHFSA, Associate Professor of Medicine, Chief, Heart Failure and Transplant, MUSC Health. Accessed at: https://www.acponline.org/system/files/documents/about_acp/chapters/sc/18mtg/tedford.pdf
- Two new drugs added to heart failure guidelines, By American Heart Association News. May 20, 2016. ©2020 American Heart Association, Inc. All rights reserved. Accessed at: https://www.heart.org/en/news/2018/05/01/two-new-drugs-added-to-heart-failure-guidelines
- Novartis Entresto Journey: Hindsight is 20/20, by Lucy Ellis. Published online May 15, 2017. Scrip Pharma Intelligence. Accessed at; https://realendpoints.com/wp-content/uploads/2018/02/SC098679_Novartis-Entresto-Voyage.pdf
- Sacubitril-Valsartan Real-World Assessment of Total Cost of Care and Resource Utilization Pre/Post Initiation Among Commercially Insured Members with Reduced Ejection Fraction Heart Failure. Oct 2020 © 2020 Prime Therapeutics LLC, Accessed on Decenber 3, 2020. https://www.primetherapeutics.com/content/dam/corporate/Documents/Newsroom/Pressreleases/2020/document-2020-amcp-entresto.pdf
- Study shows taking Entresto® as prescribed associated with lowering total cost of care by $6.7 million, Prime Therapeutics. Oct. 20, 2020. © 2020 Prime Therapeutics LLC, Accessed at: https://www.primetherapeutics.com/en/news/pressreleases/2020/release-2020-amcp-entresto.html
- AMA. Measuring progress in improving prior authorization. https://www.ama-assn.org/system/files/2019-03/prior-auth-survey.pdf,
Related news
Perspectives
April 25, 2024
Drug Approvals Monthly Update: April 2024
This monthly update of United States (U.S.) Food and Drug Administration (FDA) approvals…
Perspectives
April 24, 2024
Prime/MRx resident wins AMCP Foundation Best Poster Award
Ai Quynh Nguyen, PharmD, was recently recognized for her research on opioid-prescribing patterns and outcomes
Perspectives
April 23, 2024
Expert Clinical Network Insights: April 2024
A look into our Expert Clinical Network (ECN) – part of Prime/MRx’s value-based approach to medical and pharmacy benefit management that offers access to more than 175 national and world-renowned key opinion leaders in multiple disease categories who provide expertise on challenging prior authorization case reviews, peer-to-peer discussions, drug policy development and formulary guidance