Perspectives
Cellular therapies continue to evolve
CAR-T cell therapy: supercharged to become cancer killers.
June 6, 2020Can you hear me now?
It’s loud, and proud and it has arrived. Chimeric antigen receptor T cell therapy (CAR-T). Two CAR-T products are already on the market. Several have PDUFA dates set within the next year, dozens more are in Phase 3 testing, and hundreds more are close behind.
Cell therapy dates back to the first blood transfusions in the 1940s. Then there were the first organ and bone marrow transplantations in the 1960s and 1970s.2 Cellular therapy is the infusion or transplantation of human cells to treat an inherited or acquired disease, or to replace or repair damaged tissue and/or cells.1
This article will focus mainly on CAR-T cell therapy.
CAR-T is an important new treatment option for certain lymphoma and leukemia patients who have relapsed or become treatment resistant. CAR-T cell therapy uses a patient’s own immune cells. Those immune cells are removed and re-engineered in the lab. When the modified cells are infused back into the patient, they’re primed to seek out and kill cancer cells.3
A look at the CAR-T treatment process3
Although the treatment is given in a single infusion, preparing it takes several weeks. A CAR-T treatment includes:
- Leukapheresis: The patient is first admitted to a specialized medical center approved to conduct the procedure. A team removes whole blood from the patient. T-cells are separated and the rest of the blood cells and plasma is returned back into the patient’s bloodstream. The separated T-cells are frozen and shipped to a specialized lab.
- Production and replication of CAR-T cells: At the lab, the T cells are genetically engineered to produce chimeric antigen receptors (CAR) that attach to the T cell surface. This process converts the T cells into cancer-killing cells, which are then grown until they number in the billions. This takes about three weeks.
- Lymphodepleting/conditioning chemotherapy: While the CAR-T cells are growing in the laboratory, the patient is given chemotherapy. This helps to create a favorable environment, so when the CAR-T cells are infused, they will continue to replicate while in the patient’s body.
- Re-infusion of cells: The modified cells are re-infused into the patient. The CAR-T cells target the patient’s cancer cells and begin to destroy them.
- Patient is monitored for adverse events: After the treatment is infused, patients may need to be hospitalized due to side effects like cytokine release syndrome (CRS). Although it’s a known and serious complication of CAR-T cell therapy, getting CRS can be a good indication that the therapy is working.)
Cell therapies on the market
Kymriah® (tisagenlecleucel) from Novartis was the first CAR-T cell therapy available. Kymriah received FDA approval in August 2017 for the treatment of acute lymphoblastic leukemia.
Yescarta® (axicabtagene ciloleucel) from Kite followed in October 2017, and was the first to be approved for the treatment of adults with large B cell lymphoma.
Kymriah received FDA approval for the additional indication to treat large B cell lymphoma in May 2018.
In May 2020, the Moffitt Cancer Center (MCC) conducted a retrospective analysis that suggests that patients do not need to meet Yescarta’s clinical trials’ eligibility criteria including upper age limits and those with underlying conditions to benefit from the drug. For the study, the MCC pooled retrospective data on 298 patients across 16 cancer treatment facilities.4
FDA Approved CAR-T Cell Therapy
CAR-T research expands
Currently, CAR-T cell therapy is limited in two ways. At this time, CAR-T is effective on liquid tumors found in body fluids. Lymphomas and leukemias are examples of liquid tumors. Second, CAR-T cell therapy is also limited to patients who have relapsed from other kinds of treatment, or who are treatment resistant. This means the current CAR-T cell therapy market is targeting less than 5 percent of cancer patients today.5
This pipeline of investigative CAR-T cell therapies has rapidly expanded to more than 500 trials that are underway in 2019.6 CAR-T cell therapies are evolving past targeting CD-19, to a wider range of targets and tumor types. (CD-19 has been a reliable biomarker for these blood cancers, but it is not the only one.)
According to Janet Lambert, CEO of the Alliance for Regenerative Medicine (ARM), “The indications targeted range from blood cancers to myeloma, melanoma, cervical cancer and more. Many of these therapies are showing the same kind of outstanding clinical trial results that we showed in approved therapies.”6
Cell Therapy 2020 Pipeline
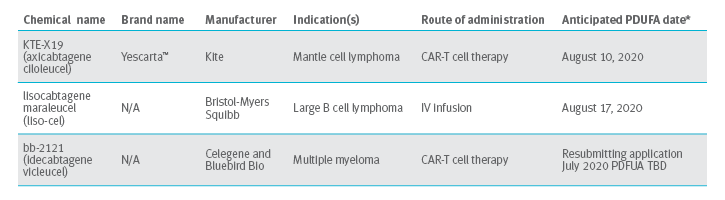
CAR-T cell therapy: version 2.0 in development
Cellular therapies that attack lymphoma demonstrate much promise, so scientists continue to build on CAR-T cell technology. Scientist hope to treat even more difficult, diverse and resistant cancers.
Resistant leukemia: For example, Texas Children’s Hospital is engineering a CAR-T cell that targets three antigens in hopes of defeating resistant leukemia. Currently, CAR-T just targets CD-19. The new CAR-T cells would target CD-19, CD-20 and CD-22.7
B cell malignancies: The FDA is reviewing MorphoSys’ Biologics License Application (BLA) for tafasitamab (MOR208), a humanized monoclonal antibody. This is directed against CD-19 for the treatment of B cell malignancies such as non-Hodgkin’s lymphoma, small lymphocytic lymphoma and chronic lymphocytic leukemia. MorphoSys is working toward getting a PDUFA date in in 2022.8,9
CAR-T cell therapy and solid tumors
Solid tumors are a larger market for CAR-T cell therapy. Solid tumors are a mass of cells that do not contain cysts or liquid. Solid tumors present a challenge in three different ways:10
- Finding the tumor
- Entering the tumor
- Surviving in the tumor
Solutions being pursued
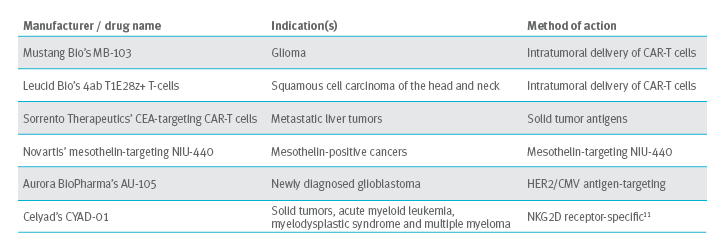
CAR-T cell therapies show great potential. We are working with our Blue Plan clients to overcome barriers that may hinder commercial success. Follow our pipeline and look on the Prime Insights pages for more articles on managing this challenging future.
References
1. http://www.aabb.org/aabbcct/therapyfacts/Pages/default.aspx
2. https://annualmeeting.asgct.org/about_gene_therapy/genevscell.php
3. LLS.org Chimeric antigen receptor (CAR) T-cell therapy. Accessed in June 2017 at: https://www.lls.org/treatment/types-of-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy.
4. https://www.newswise.com/articles/moffitt-cancer-center-study-suggests-more-could-benefit-from-car-t-cell-therapy
5. Surveillance, Epidemiology, and End Results program (SEER) research data (1975–2016), National Cancer Institute, November 2018, seer.cancer.gov.
6. https://alliancerm.org/sector-report/2019-annual-report/
7. https://www.chla.org/research/could-car-t-version-20-be-the-horizon
8. https://www.morphosys.com/pipeline/proprietary-portfolio/tafasitamab-mor208
9. https://www.fiercebiotech.com/biotech/incyte-pays-750m-upfront-for-rights-to-morphosys-cancer-drug
10. https://www.frontiersin.org/articles/10.3389/fimmu.2019.00128/full
11. https://www.cellandgene.com/doc/car-t-cell-therapies-current-limitations-future-opportunities-0001
Related news
Perspectives
April 25, 2024
Drug Approvals Monthly Update: April 2024
This monthly update of United States (U.S.) Food and Drug Administration (FDA) approvals…
Perspectives
April 24, 2024
Prime/MRx resident wins AMCP Foundation Best Poster Award
Ai Quynh Nguyen, PharmD, was recently recognized for her research on opioid-prescribing patterns and outcomes
Perspectives
April 23, 2024
Expert Clinical Network Insights: April 2024
A look into our Expert Clinical Network (ECN) – part of Prime/MRx’s value-based approach to medical and pharmacy benefit management that offers access to more than 175 national and world-renowned key opinion leaders in multiple disease categories who provide expertise on challenging prior authorization case reviews, peer-to-peer discussions, drug policy development and formulary guidance
