Perspectives
Biosimilars – overcoming brand bias and misperceptions
Can we move the neutropenia market to biosimilars?
December 3, 2018 starts in the body, how and where it grows, speed of growth, lifestyle and genetics – just to name a few factors. This makes it so important for us to be patient-centered in our approach. It’s not like high blood pressure, where guidelines are driven by a single set of metrics.”
starts in the body, how and where it grows, speed of growth, lifestyle and genetics – just to name a few factors. This makes it so important for us to be patient-centered in our approach. It’s not like high blood pressure, where guidelines are driven by a single set of metrics.”
Between 2011 and 2016, 58 new drugs were launched to treat 22 cancer indications. Another 600 oncology drugs are in the pipeline.¹ Most cancer drugs approved after 2009 were priced at more than $100,000/year. Some exceed $400,000/year.¹ ²
“Treatment choices can be really complicated,” Jeremy said. “Is the newest drug always the best? Not always, no. Is the most expensive one the best? Not always, no.”
It takes an extremely sophisticated village
Health care systems use computer models and artificial intelligence algorithms to predict therapy effects and sift through evidence. “That bigger picture can be extremely helpful,” Jeremy added. “Because no one oncologist can keep up with everything. No pharmacist can either. When it comes right down to it, a patient’s entire health care team has to work together, sharing information and working in concert to make decisions along the way,” Jeremy said.
“When it comes right down to it, it’s one patient at a time, one day at a time, with his or her care team.”
Jeremy’s nine years managing an oncology clinic gave him real world practice management experience. He brought the immediacy of those years on a patient care team to Prime. “We bring together the team at Prime – clinical, rebate, client engagement, health outcomes, network, specialty – and we meet with the medical and pharmacy directors of our health care clients,” Jeremy said. “We keep the patient front and center as we make benefit design and formulary decisions, just as we would if we were in a clinic setting with the patient.”
Most patients with cancer need treatment for neutropenia
Most patients with cancer develop neutropenia (a low level of white blood cells), usually due to chemotherapy. Patients and their doctors now have treatment choices for neutropenia that can give patients and health plans significant savings.
 Granulocyte-colony stimulating factor (G-CSF) drugs treat neutropenia: These drugs emulate the body’s own G-CSF to help patients with cancer replace white blood cells depleted by chemotherapy.
Granulocyte-colony stimulating factor (G-CSF) drugs treat neutropenia: These drugs emulate the body’s own G-CSF to help patients with cancer replace white blood cells depleted by chemotherapy.
Until recently, patients have had two options for treating neutropenia. Pegfilgrastim (Neulasta®) and filgrastim (Neupogen®) Both drugs are both granulocyte-colony stimulating factor (G-CSF), which stimulate the growth of white blood cells. The first is long acting, the second short acting.
“Now we have biosimilar options for both short-acting and longer-lasting blood growth factors,” Jeremy pointed out. “This gives us a tremendous opportunity to help our health plan clients save millions of dollars and still deliver the highest quality of care for patients.”
The challenge – overcoming brand bias and misperceptions
Biosimilars have been on the horizon for a long time. But they have not made a dent in market share for any drug categories in the United States. There are a number of complex reasons.³
In other drug classes where a biosimilar has been approved, some pharmaceutical companies have used rebate strategies that incent patients to stay on brand-name drugs when a lower-cost but equally effective biosimilar becomes available. That’s not the issue with blood growth factor, since one manufacturer dominates the brand-name side.
Drugs that treat neutropenia
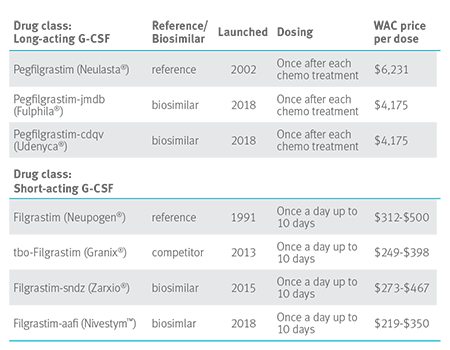
Pegflgrastim biosimilar pipeline for 2019 also includes Lapelga™ and LA-EP2006. This will create further downward price pressure in this drug class.
“Here we have an opportunity in a small drug class, with one dominant brand name, to help move the market to a biosimilar,” Jeremy explained. “This will help plans and members save significant money.”
If 75 percent of Blue Plan members taking Neulasta® switched to Fulphila®, the book of business savings would be $50 million (approximately 1,400 of 1,900 Neulasta prescriptions).
A new day for biosimilars in the United States
This brings us back to those 600 oncology drugs under development.
“As specialty drugs march towards 60 percent of total drug spend, Biosimilars can give us a ‘brake,’” Jeremy quipped. “For our members to get the drugs they need at affordable costs, we need to help the market accept biosimilars as therapeutic equivalents.”
References
- The High Cost of Cancer Treatment: Avoiding financial disaster can add stress to patient’s battle against the disease, by Peter Moore, AARP The Magazine, June/July 2018. AARP. https://www.aarp.org/money/credit-loans-debt/info-2018/the-high-cost-of-cancer-treatment.html
- “The Imperative of Addressing Cancer Drug Costs and Value,” by Barbara K. Rimer, Dr.P.H. March 15, 2018. National Institutes of Health … Turning Discovery into Health.® Accessed at: https://www.cancer.gov/news-events/cancer-currents-blog/2018/presidents-cancer-panel-drug-prices
- “New report on the market potential of biosimilars,” by Prime Therapeutics, Feb. 2015. Accessed at: https://www.primetherapeutics.com/en/news/prime-insights/2015-insights/2015-02-24-biosimilars.html
May 2017 Global Oncology Trends 2017: Advances, complexities and cost. by Quintiles IMS Institute. Global Oncology Trends 2017. Report by the QuintilesIMS Institute. Accessed at: https://morningconsult.com/wp-content/uploads/2017/06/QuintilesIMS-Institute-Oncology-Report.pdf
Management of Neutropenia in Cancer Patients. Maryam B. Lustberg, MD, MPH. Clin Adv Hematol Oncol. 2012 Dec; 10(12): 825–826. Accessed at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4059501/
Related news
Perspectives
April 24, 2024
Prime/MRx resident wins AMCP Foundation Best Poster Award
Ai Quynh Nguyen, PharmD, was recently recognized for her research on opioid-prescribing patterns and outcomes
Perspectives
April 23, 2024
Expert Clinical Network Insights: April 2024
A look into our Expert Clinical Network (ECN) – part of Prime/MRx’s value-based approach to medical and pharmacy benefit management that offers access to more than 175 national and world-renowned key opinion leaders in multiple disease categories who provide expertise on challenging prior authorization case reviews, peer-to-peer discussions, drug policy development and formulary guidance
Perspectives
April 23, 2024
April Fraud Focus – How to prioritize drug safety at home
While ensuring drug safety is often considered the responsibility of pharmaceutical industry players,…