Perspectives
Gene therapy: A promising future at a high price
Gene therapy harnesses the body’s capacity to heal itself
March 10, 2022- Replacing a disease-causing gene with a healthy copy of the gene
- Inactivating a disease-causing gene that is not functioning properly
- Introducing a new or modified gene into the body to help treat a disease1
The idea for gene therapy was first published in 1972, but practical applications weren’t approved in the United States until 2017.
How is gene therapy delivered?
One of the most common ways gene therapy is delivered is through viral vectors — a virus that is modified in the laboratory to carry genetic material into cells without causing an infection.
Definition: Viruses are germs, designed to invade living, normal cells and then multiply to cause infectious disease. Gene therapy uses that natural ability to deliver genetic material. Before a virus can be used to carry therapeutic genes, it must be modified. It is stripped of the parts that can cause an infectious disease. Then it becomes a viral vector.2
What are the applications for gene therapy?
Gene therapy can be applied in a number of areas including:
- Oncology (cancer)
- Musculoskeletal
- Central nervous system
- Endocrine, metabolic and genetic disorders
- Cardiovascular
- Hematology
- Ophthalmology
- Immunology and inflammation
- Infectious diseases
- Dermatology
- Gastroenterology
- Respiratory
- Lymphatic, and more
Gene therapies do not always completely cure a disease, but in many cases may improve patient outcomes. Although gene therapy demonstrates a promising future, we are still learning a lot about it and its durability (how long its effects will last in the body).

Gene therapy has a strong outlook
The U.S. Food and Drug Administration (FDA) anticipates that each year they will receive more than 200 investigational new drug (IND) applications for new gene therapy. By 2025, the FDA expects to approve 10 to 20 new gene and cell therapies per year.2
Total of 352 clinical trials underway in Q4 2019
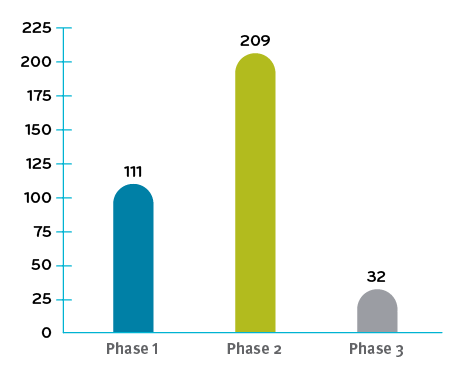
Clinical data numbers for chart were referenced from Alliance for Regenerative Medicine3
Gene therapy costs are high
Gene therapies are complex and difficult to produce. They often target diseases with small populations.4 Together, these factors make gene therapy treatments extremely expensive.
The FDA has approved two gene therapies.
Luxturna®: In December 2017, the FDA approved Spark Therapeutics’ Luxturna (voretigene neparvovec-rzyl)5 for the treatment of confirmed biallelic RPE65 mutation-associated retinal dystrophy. It is administered as two separate subretinal injections, one per eye. The wholesale acquisition cost (WAC) of Luxturna is $850,000 per person or $425,000 per eye, not including medical costs.
Zolgensma®: AveXis’ Zolgensma® (onasemnogene abeparvovec-xioi), is FDA approved for the treatment of spinal muscular atrophy (SMA) in patients under the age of two years old. The WAC for a one-time intravenous infusion of Zolgensma is $2.125 million per treated patient.6
Current approved gene therapies and 2022 anticipated approvals7
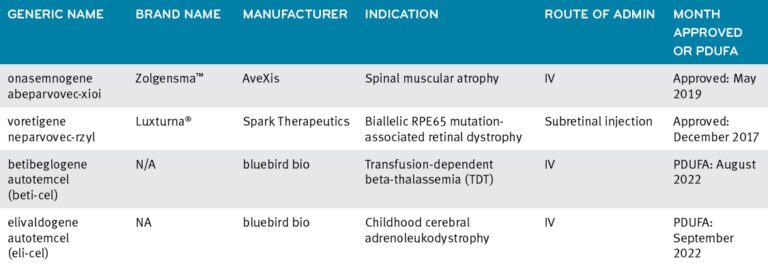
Definition: PDFUA stands for the Prescription Drug User Fee Act (PDUFA), created by Congress in 1992. It authorizes FDA to collect user fees from drug companies as part of expediting the drug approval process. Over time, the acronym and shorthand, PDUFA date, has come to refer to the scheduled date when the FDA will announce its decision on whether a drug is approved or not.
PreserveRxSM will help support Blue Plans
An estimated 60 gene therapies will come to market in the next 10 years. PreserveRx offers health plans financial protection, helping to preserve patient access to these therapies. Prime, together with BCS Insurance Company, offers PreserveRx, a robust gene therapy reinsurance.
PreserveRx can protect both health plans from sudden, one-time costs due to ultra-expensive gene therapies. Currently PreserveRx covers Luxturna® and Zolgensma® and will be expanded to include additional FDA-approved gene therapies.
Prime keeps clients ahead of the drug pipeline
Prime posts new drug approvals in its monthly pipeline updates. Prime also posts pipeline projections quarterly, in its March, June, September and December specialty pipeline updates.
References
- “What is Gene Therapy?” U.S. Food & Drug Administration. Accessed 4/7/20: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
- https://medlineplus.gov/viralinfections.html
- FDA Statement from Commissioner of Food and Drug Administration Scott Gottlieb M.D. on Jan. 19, 2019. Accessed 4/7/20 at: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics
- “Advancing Gene, Cell, & Tissue-Based Therapies.” Alliance for Regenerative Medicine 2019 Annual Report. Accessed April 7, 2020. https://alliancerm.org/publication/2019-annual-report/
- FDA Approval Letter. Accessed 4/7/20 at: https://www.fda.gov/media/109487/download
- “At Over $2 Million Zolgensma Is The World’s Most Expensive Therapy, Yet Relatively Cost-Effective,” by Joshua Cohen. Forbes. June 5, 2019. Accessed at: https://www.forbes.com/sites/joshuacohen/2019/06/05/at-over-2-million-zolgensma-is-the-worlds-most-expensive-therapyyet-relatively-cost-effective/#655f095745f5
- bluebird Provides Update on FDA Review Timelines for Betibeglogene Autotemcel (beti-cel) for Beta-Thalassemia and Elivaldogene Autotemcel (eli-cel) for Cerebral Adrenoleukodystrophy (CALD), Jan. 18, 2022. bluebird bio. Accessed at: https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-provides-update-fda-review-timelines-betibeglogene
Related news
Perspectives
April 25, 2024
Drug Approvals Monthly Update: April 2024
This monthly update of United States (U.S.) Food and Drug Administration (FDA) approvals…
Perspectives
April 24, 2024
Prime/MRx resident wins AMCP Foundation Best Poster Award
Ai Quynh Nguyen, PharmD, was recently recognized for her research on opioid-prescribing patterns and outcomes
Perspectives
April 23, 2024
Expert Clinical Network Insights: April 2024
A look into our Expert Clinical Network (ECN) – part of Prime/MRx’s value-based approach to medical and pharmacy benefit management that offers access to more than 175 national and world-renowned key opinion leaders in multiple disease categories who provide expertise on challenging prior authorization case reviews, peer-to-peer discussions, drug policy development and formulary guidance